Identification of a Novel ERK5 (MAPK7) Inhibitor, MHJ-627, and Verification of Its Potent Anticancer Efficacy in Cervical Cancer HeLa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Chemicals
2.2. Yeast Strains, Plasmids, Growth Conditions, and Transformation
2.3. Animal Cell Lines and Culture
2.4. β-Galactosidase Reporter Assay
2.5. In Vitro Kinase Assay
2.6. Transient Transfection and qRT-PCR-Based Luciferase Reporter Assay
2.7. Quantitative Real-Time PCR Analysis
2.8. Western Blot Analysis
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. MHJ-627 Compound Synthesis
3.2. MHJ-627 Suppressed the Catalytic Activity of Mpk1 to Activate Rlm1 Transcription Factor and Attenuated the Expression of MLP1
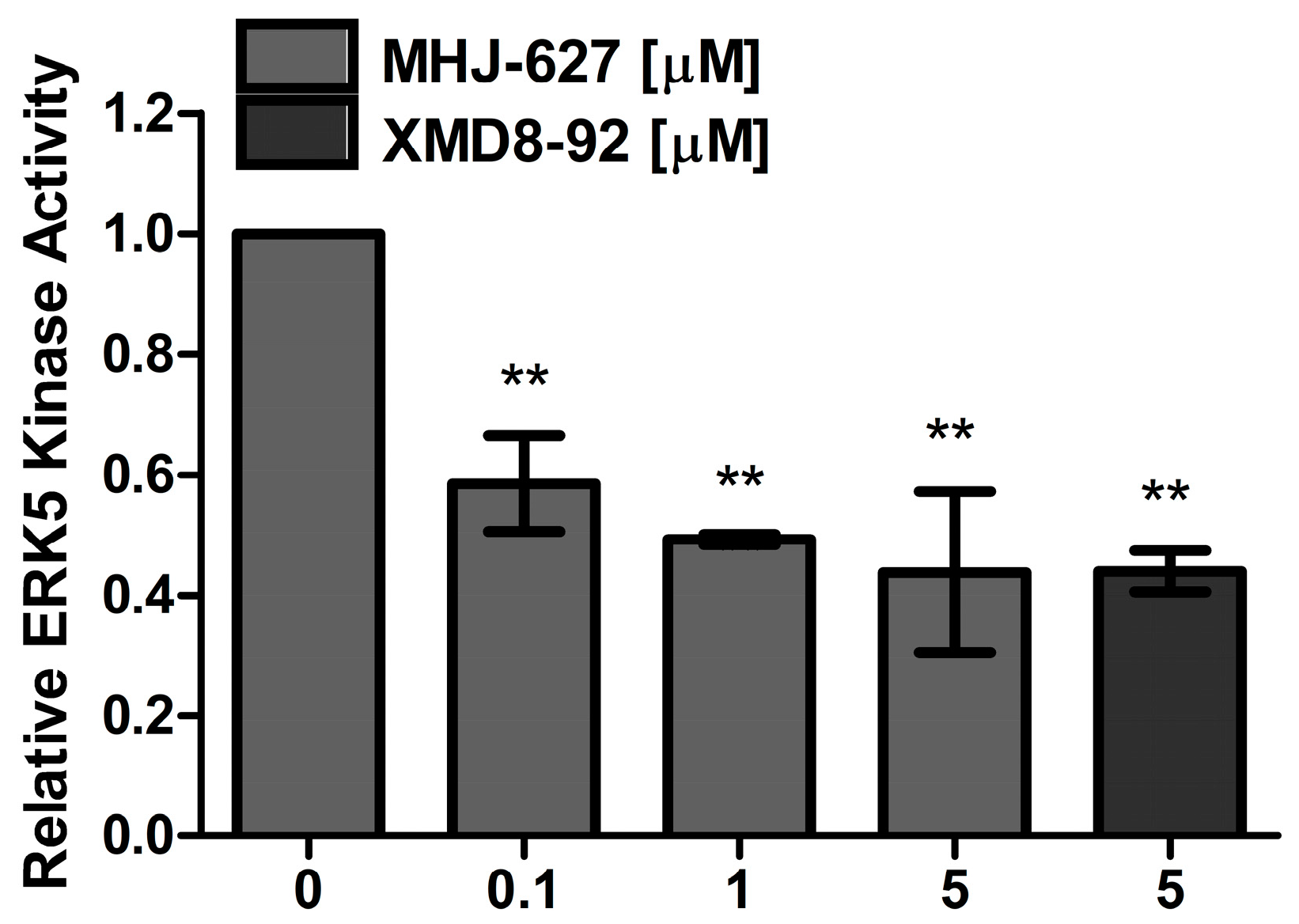
3.3. MHJ-627 Inhibited the Kinase Activity of Human ERK5 In Vitro
3.4. MHJ-627 Suppressed the Activity of ERK5 and Impaired AP-1 Activity in HeLa Cells
3.5. ERK5 Inhibition by MHJ-627 Modified the mRNA Expression of Genes Regulated by ERK5
3.6. MHJ-627 Paradoxically Increased ERK5 Expression Possibly due to the Stimulatory Crosstalk of the ERK1/2 Pathway
3.7. MHJ-627 Showed Anti-Proliferative Effect in the Human Cervical Cancer HeLa Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Lemos, C.; Wortmann, L.; Eis, K.; Holton, S.J.; Boemer, U.; Moosmayer, D.; Eberspaecher, U.; Weiske, J.; Lechner, C.; et al. Discovery and characterization of the potent and highly selective (Piperidin-4-yl)pyrido[3,2-d]pyrimidine based in vitro probe BAY-885 for the kinase ERK5. J. Med. Chem. 2019, 62, 928–940. [Google Scholar] [CrossRef]
- Hayashi, M.; Lee, J.D. Role of the BMK1/ERK5 signaling pathway: Lessons from knockout mice. J. Mol. Med. 2004, 82, 800–808. [Google Scholar] [CrossRef]
- Hoang, V.T.; Yan, T.J.; Cavanaugh, J.E.; Flaherty, P.T.; Beckman, B.S.; Burow, M.E. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017, 392, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Campbell, D.G.; Morrice, N.; Peggie, M.; Cohen, P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem. J. 2003, 372, 567–575. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.; Müller, J.; Cross, M.J. ERK5: Structure, regulation and function. Cell. Signal. 2012, 24, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Buschbeck, M.; Ullrich, A. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J. Biol. Chem. 2005, 280, 2659–2667. [Google Scholar] [CrossRef]
- Nishimoto, S.; Nishida, E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006, 7, 782–786. [Google Scholar] [CrossRef]
- Morimoto, H.; Kondoh, K.; Nishimoto, S.; Terasawa, K.; Nishida, E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J. Biol. Chem. 2007, 282, 35449–35456. [Google Scholar] [CrossRef]
- Stecca, B.; Rovida, E. Impact of ERK5 on the hallmarks of cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef]
- Monti, M.; Celli, J.; Missale, F.; Cersosimo, F.; Russo, M.; Belloni, E.; Di Matteo, A.; Lonardi, S.; Vermi, W.; Ghigna, C.; et al. Clinical significance and regulation of ERK5 expression and function in cancer. Cancers 2022, 14, 348. [Google Scholar] [CrossRef]
- Gavine, P.R.; Wang, M.; Yu, D.; Hu, E.; Huang, C.; Xia, J.; Su, X.; Fan, J.; Zhang, T.; Ye, Q.; et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer 2015, 15, 454. [Google Scholar] [CrossRef]
- Shukla, A.; Miller, J.M.; Cason, C.; Sayan, M.; MacPherson, M.B.; Beuschel, S.L.; Hillegass, J.; Vacek, P.M.; Pass, H.I.; Mossman, B.T. Extracellular signal-regulated kinase 5: A potential therapeutic target for malignant mesotheliomas. Clin. Cancer Res. 2013, 19, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Tapping, R.I.; Huang, S.; Watson, M.H.; Ulevitch, R.J.; Lee, J.D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 1998, 395, 713–716. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, J.; Luo, S.; Yi, J.; Wang, P.; Zheng, Q.; Wen, Y. miR-143 is associated with proliferation and apoptosis involving ERK5 in HeLa cells. Oncol. Lett. 2016, 12, 3021–3027. [Google Scholar] [CrossRef]
- Simões, A.E.; Rodrigues, C.M.; Borralho, P.M. The MEK5/ERK5 signalling pathway in cancer: A promising novel therapeutic target. Drug Discov. Today 2016, 21, 1654–1663. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Truman, A.W.; Millson, S.H.; Nuttall, J.M.; King, V.; Mollapour, M.; Prodromou, C.; Pearl, L.H.; Piper, P.W. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot. Cell 2006, 5, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Plovins, A.; Martín, H.; Molina, M.; Nombela, C. Characterization of domains in the yeast MAP kinase Slt2 (Mpk1) required for functional activity and in vivo interaction with protein kinases Mkk1 and Mkk2. Mol. Microbiol. 1995, 17, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.G.; Lee, H.; Lee, T.H.; Kim, K.Y.; Kim, H. Antifungal activity of 1,4-Dialkoxynaphthalen-2-Acyl imidazolium salts by inducing apoptosis of pathogenic Candida spp. Pharmaceutics 2021, 13, 312. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, Y.; Moon, H.; Lee, E.H.; Lee, T.H.; Kim, H. Synthesis of 1, 4-Dialkoxynaphthalene-Based Imidazolium salts and their cytotoxicity in cancer cell lines. Int. J. Mol. Sci. 2023, 24, 2713. [Google Scholar] [CrossRef]
- Kim, K.Y.; Truman, A.W.; Levin, D.E. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol. Cell. Biol. 2008, 28, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Meth. Enzymol. 1983, 101, 181–191. [Google Scholar] [CrossRef]
- Rose, M.; Botstein, D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Meth. Enzymol. 1983, 101, 167–180. [Google Scholar] [CrossRef]
- Stahl, G.; Salem, S.N.; Chen, L.; Zhao, B.; Farabaugh, P.J. Translational accuracy during exponential, postdiauxic, and stationary growth phases in Saccharomyces cerevisiae. Eukaryot. Cell 2004, 3, 331–338. [Google Scholar] [CrossRef]
- Cude, K.; Wang, Y.; Choi, H.J.; Hsuan, S.L.; Zhang, H.; Wang, C.Y.; Xia, Z. Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J. Cell Biol. 2007, 177, 253–264. [Google Scholar] [CrossRef]
- Lin, E.C.; Amantea, C.M.; Nomanbhoy, T.K.; Weissig, H.; Ishiyama, J.; Hu, Y.; Sidique, S.; Li, B.; Kozarich, J.W.; Rosenblum, J.S. ERK5 kinase activity is dispensable for cellular immune response and proliferation. Proc. Natl. Acad. Sci. USA. 2016, 113, 11865–11870. [Google Scholar] [CrossRef]
- Siano, G.; Caiazza, M.C.; Ollà, I.; Varisco, M.; Madaro, G.; Quercioli, V.; Calvello, M.; Cattaneo, A.; Di Primio, C. Identification of an ERK inhibitor as a therapeutic drug against Tau aggregation in a New Cell-Based Assay. Front. Cell. Neurosci. 2019, 13, 386. [Google Scholar] [CrossRef]
- Arias, P.; Díez-Muñiz, S.; García, R.; Nombela, C.; Rodríguez-Peña, J.M.; Arroyo, J. Genome-wide survey of yeast mutations leading to activation of the yeast cell integrity MAPK pathway: Novel insights into diverse MAPK outcomes. BMC Genom. 2011, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M. Biotechnology: A Laboratory Course, 1st ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 129–132. [Google Scholar]
- Kang, C.; Kim, J.S.; Kim, C.Y.; Kim, E.Y.; Chung, H.M. The pharmacological inhibition of ERK5 enhances apoptosis in acute myeloid leukemia cells. Int. J. Stem Cell 2018, 11, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jung, U.S.; Garrett-Engele, P.; Roe, T.; Cyert, M.S.; Levin, D.E. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 1998, 18, 1013–1022. [Google Scholar] [CrossRef]
- Myers, S.M.; Bawn, R.H.; Bisset, L.C.; Blackburn, T.J.; Cottyn, B.; Molyneux, L.; Wong, A.C.; Cano, C.; Clegg, W.; Harrington, R.W.; et al. High-throughput screening and hit validation of extracellular-related kinase 5 (ERK5) inhibitors. ACS Comb. Sci. 2016, 18, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Erazo, T.; Lizcano, J.M. ERK5 and cell proliferation: Nuclear localization is what matters. Front. Cell Dev. Biol. 2016, 4, 105. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kim, K.Y. Inhibition of proinflammatory cytokines in Cutibacterium acnes-induced inflammation in HaCaT cells by using Buddleja davidii aqueous extract. Int. J. Inflam. 2020, 2020, 8063289. [Google Scholar] [CrossRef]
- Yamada, Y.; Watanabe, Y.; Zhang, J.; Haraoka, J.; Ito, H. Changes in cortical and cerebellar bcl-2 mRNA levels in the developing hydrocephalic rat (LEW-HYR) as measured by a real time quantified RT-PCR. Neuroscience 2002, 114, 165–171. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.G.; Kim, K.Y. Trichosanthes kirilowii Extract Promotes Wound Healing through the Phosphorylation of ERK1/2 in Keratinocytes. Biomimetics 2022, 7, 154. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Shin, Y.K.; Kim, K.Y. Gentisic acid stimulates Keratinocyte proliferation through ERK1/2 phosphorylation. Int. J. Med. Sci. 2020, 17, 626–631. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Q.Y.; Huang, H.Q. The evidence of HeLa cell apoptosis induced with tetraethylammonium using proteomics and various analytical methods. J. Biol. Chem. 2014, 289, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Pirkmajer, S.; Chibalin, A.V. Serum starvation: Caveat emptor. Am. J. Physiol. Cell Physiol. 2011, 301, C272–C279. [Google Scholar] [CrossRef] [PubMed]
- Mbeunkui, F.; Fodstad, O.; Pannell, L.K. Secretory protein enrichment and analysis: An optimized approach applied on cancer cell lines using 2D LC-MS/MS. J. Proteome Res. 2006, 5, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Pirt, S.J. Growth of human diploid cells (strain MRC-5) in defined medium; replacement of serum by a fraction of serum ultrafiltrate. J. Cell. Sci. 1979, 35, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Colzani, M.; Waridel, P.; Laurent, J.; Faes, E.; Rüegg, C.; Quadroni, M. Metabolic labeling and protein linearization technology allow the study of proteins secreted by cultured cells in serum-containing media. J. Proteome Res. 2009, 8, 4779–4788. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Kim, J.G.; Shin, Y.K.; Kim, K.Y. Antimicrobial activity of 4-hydroxyderricin, sophoraflavanone G, acetylshikonin, and kurarinone against the bee pathogenic bacteria Paenibacillus larvae and Melissococcus plutonius. J. Apic. Res. 2021, 60, 118–122. [Google Scholar] [CrossRef]
- Gao, L.; Fei, J.; Zhao, J.; Li, H.; Cui, Y.; Li, J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012, 6, 8030–8040. [Google Scholar] [CrossRef]
- Kim, J.; Shin, Y.K.; Kim, K.Y. Promotion of Keratinocyte proliferation by Tracheloside through ERK1/2 stimulation. Evid. Based Complement. Altern. Med. 2018, 2018, 4580627. [Google Scholar] [CrossRef]
- Samsuzzaman, M.; Lee, J.H.; Moon, H.; Lee, J.; Lee, H.; Lim, Y.; Park, M.G.; Kim, H.; Kim, S.Y. Identification of a potent NAFLD drug candidate for controlling T2DM-mediated inflammation and secondary damage in vitro and in vivo. Front. Pharmacol. 2022, 13, 943879. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, X.; Lu, B.; Cameron, M.; Fearns, C.; Patricelli, M.P.; Yates, J.R., 3rd; Gray, N.S.; Lee, J.D. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell. 2010, 18, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.J.; Tucker, J.A.; Lochhead, P.A. Small molecule ERK5 kinase inhibitors paradoxically activate ERK5 signalling: Be careful what you wish for. Biochem. Soc. Trans. 2020, 48, 1859–1875. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Ozanne, B.W.; Spence, H.J.; McGarry, L.C.; Hennigan, R.F. Invasion is a genetic program regulated by transcription factors. Curr. Opin. Genet. Dev. 2006, 16, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.M.; Wang, J.; Deng, X.; Pattison, M.J.; Arthur, J.S.; Erazo, T.; Gomez, N.; Lizcano, J.M.; Gray, N.S.; Knapp, S. X-ray crystal structure of ERK5 (MAPK7) in complex with a specific inhibitor. J. Med. Chem. 2013, 56, 4413–4421. [Google Scholar] [CrossRef]
- Schweppe, R.E.; Cheung, T.H.; Ahn, N.G. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J. Biol. Chem. 2006, 281, 20993–21003. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, D.; Gao, W.; Liao, Z.; Chen, Y.; Zhang, S.; Zhou, H.; Su, P.; Xu, C. Conditional ablation of MAPK7 expression in chondrocytes impairs endochondral bone formation in limbs and adaptation of chondrocytes to hypoxia. Cell Biosci. 2020, 10, 103. [Google Scholar] [CrossRef]
- Tubita, A.; Lombardi, Z.; Tusa, I.; Lazzeretti, A.; Sgrignani, G.; Papini, D.; Menconi, A.; Gagliardi, S.; Lulli, M.; Dello Sbarba, P.; et al. Inhibition of ERK5 elicits cellular senescence in melanoma via the cyclin-dependent kinase inhibitor p21. Cancer Res. 2022, 82, 447–457. [Google Scholar] [CrossRef]
- Kelman, Z. PCNA: Structure, functions and interactions. Oncogene 1997, 14, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Sofer, A.; Lei, K.; Johannessen, C.M.; Ellisen, L.W. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol. 2005, 25, 5834–5845. [Google Scholar] [CrossRef]
- Wang, L.; Kong, W.; Liu, B.; Zhang, X. Proliferating cell nuclear antigen promotes cell proliferation and tumorigenesis by up-regulating STAT3 in non-small cell lung cancer. Biomed. Pharmacother. 2018, 104, 595–602. [Google Scholar] [CrossRef]
- Lu, E.M.; Ratnayake, J.; Rich, A.M. Assessment of proliferating cell nuclear antigen (PCNA) expression at the invading front of oral squamous cell carcinoma. BMC Oral. Health 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Gopal, P.; Lim, S.; Wei, X.; Chandramohan, A.; Mangadu, R.; Smith, J.; Ng, S.; Gindy, M.; Phan, U.; et al. Targeted degradation of PCNA outperforms stoichiometric inhibition to result in programed cell death. Cell Chem. Biol. 2022, 29, 1601–1615.e7. [Google Scholar] [CrossRef] [PubMed]
- Zammarchi, F.; Morelli, M.; Menicagli, M.; Di Cristofano, C.; Zavaglia, K.; Paolucci, A.; Campani, D.; Aretini, P.; Boggi, U.; Mosca, F.; et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Clin. Pathol. 2011, 178, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hisamuddin, I.M.; Nandan, M.O.; Babbin, B.A.; Lamb, N.E.; Yang, V.W. Identification of Krüppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene 2004, 23, 395–402. [Google Scholar] [CrossRef]
- Hu, W.; Hofstetter, W.L.; Li, H.; Zhou, Y.; He, Y.; Pataer, A.; Wang, L.; Xie, K.; Swisher, S.G.; Fang, B. Putative tumor-suppressive function of Krüppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 2009, 15, 5688–5695. [Google Scholar] [CrossRef]
- Yang, W.T.; Zheng, P.S. Promoter hypermethylation of KLF4 inactivates its tumor suppressor function in cervical carcinogenesis. PLoS ONE 2014, 9, e88827. [Google Scholar] [CrossRef]
- Xiong, G.; Xu, R. Retinoid orphan nuclear receptor alpha (RORα) suppresses the epithelial-mesenchymal transition (EMT) by directly repressing Snail transcription. J. Biol. Chem. 2022, 298, 102059. [Google Scholar] [CrossRef]
- Du, J.; Xu, R. RORα, a potential tumor suppressor and therapeutic target of breast cancer. Int. J. Mol. Sci. 2012, 13, 15755–15766. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Ross, K.N.; Lander, E.S.; Golub, T.R. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003, 33, 49–54. [Google Scholar] [CrossRef]
- Alexopoulou, A.N.; Leao, M.; Caballero, O.L.; Da Silva, L.; Reid, L.; Lakhani, S.R.; Simpson, A.J.; Marshall, J.F.; Neville, A.M.; Jat, P.S. Dissecting the transcriptional networks underlying breast cancer: NR4A1 reduces the migration of normal and breast cancer cell lines. Breast Cancer Res. 2010, 12, R51. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Weygant, N.; Qu, D.; Chandrakesan, P.; Bannerman-Menson, E.; Ali, N.; Pantazis, P.; Westphalen, C.B.; Wang, T.C.; et al. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014, 351, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, Y.; Liu, Y.; Sun, D.; Zhen, Y.; Liu, J.; Fu, L.; Zhang, L.; Ouyang, L. Discovery of a novel dual-target inhibitor of ERK1 and ERK5 that induces regulated cell death to overcome compensatory mechanism in specific tumor types. J. Med. Chem. 2020, 63, 3976–3995. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Chen, X.C.; Yang, G.Y.; Zhou, L.F. U0126 prevents ERK pathway phosphorylation and interleukin-1beta mRNA production after cerebral ischemia. Chin. Med. Sci. J. 2004, 19, 270–275. [Google Scholar]
- Tubita, A.; Tusa, I.; Rovida, E. Playing the Whack-A-Mole game: ERK5 activation emerges among the resistance mechanisms to RAF-MEK1/2-ERK1/2- targeted therapy. Front. Cell Dev. Biol. 2021, 9, 647311. [Google Scholar] [CrossRef]
- de Jong, P.R.; Taniguchi, K.; Harris, A.R.; Bertin, S.; Takahashi, N.; Duong, J.; Campos, A.D.; Powis, G.; Corr, M.; Karin, M.; et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat. Commun. 2016, 7, 11551. [Google Scholar] [CrossRef]
- Cook, S.J.; Lochhead, P.A. ERK5 signalling and resistance to ERK1/2 pathway therapeutics: The path less travelled? Front. Cell Dev. Biol. 2022, 10, 839997. [Google Scholar] [CrossRef]






| Gene | Primer Sequence (5′ to 3′) | References |
|---|---|---|
| GAPDH | F: GTGAAGGTCGGAGTCAACG R: TGAGGTCAATGAAGGGGTC | [37] |
| PCNA | F: AACCTCACCAGTATGTCCAA R: ACTTTCTCCTGGTTTGGTG | [40] |
| DDIT4 | F: GTGGAGGTGGTTTGTGTATC R: CACCCCTTGCTACTCTTAC | This study |
| CXCL1 | F: AAAGCTTGCCTCAATCCTGC R: CTTCAGGAACAGCCACCAGT | This study |
| KLF4 | F: CCAATTACCCATCCTTCCTG R: CGATCGTCTTCCCCTCTTTG | This study |
| NR4A1 | F: GCTTCATGCCAGCATTATGG R: GTTCGGACAACTTCCTTCAC | This study |
| RORα | F: AGGCTCGCTAGAGGTGGTGTT R: TGAGAGTCAAAGGCACGGC | This study |
| PTPRC | F: CTTCAGTGGTCCCATTGTGGTG R: CCACTTTGTTCTCGGCTTCCAG | This study |
| CCL5 | F: TCATTGCTACTGCCCTCTGC R: TACTCCTTGATGTGGGCACG | This study |
| ICAM1 | F: AGCGGCTGACGTGTGCAGTAAT R: TCTGAGACCTCTGGCTTCGTCA | This study |
| SIGLEC1 | F: ACCTGGAGGAAACTGACAGTGG R: CTCAGTGTCACTGCCTGTCCTT | This study |
| luc2P | F: CTTTTGCAGCCCTTTCTTGC R: CTTTTGCAGCCCTTTCTTGC | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Moon, H.; Kim, H.; Kim, K.-Y. Identification of a Novel ERK5 (MAPK7) Inhibitor, MHJ-627, and Verification of Its Potent Anticancer Efficacy in Cervical Cancer HeLa Cells. Curr. Issues Mol. Biol. 2023, 45, 6154-6169. https://doi.org/10.3390/cimb45070388
Hwang J, Moon H, Kim H, Kim K-Y. Identification of a Novel ERK5 (MAPK7) Inhibitor, MHJ-627, and Verification of Its Potent Anticancer Efficacy in Cervical Cancer HeLa Cells. Current Issues in Molecular Biology. 2023; 45(7):6154-6169. https://doi.org/10.3390/cimb45070388
Chicago/Turabian StyleHwang, Jeonghye, Hyejin Moon, Hakwon Kim, and Ki-Young Kim. 2023. "Identification of a Novel ERK5 (MAPK7) Inhibitor, MHJ-627, and Verification of Its Potent Anticancer Efficacy in Cervical Cancer HeLa Cells" Current Issues in Molecular Biology 45, no. 7: 6154-6169. https://doi.org/10.3390/cimb45070388
APA StyleHwang, J., Moon, H., Kim, H., & Kim, K.-Y. (2023). Identification of a Novel ERK5 (MAPK7) Inhibitor, MHJ-627, and Verification of Its Potent Anticancer Efficacy in Cervical Cancer HeLa Cells. Current Issues in Molecular Biology, 45(7), 6154-6169. https://doi.org/10.3390/cimb45070388






