Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion
Abstract
1. Introduction
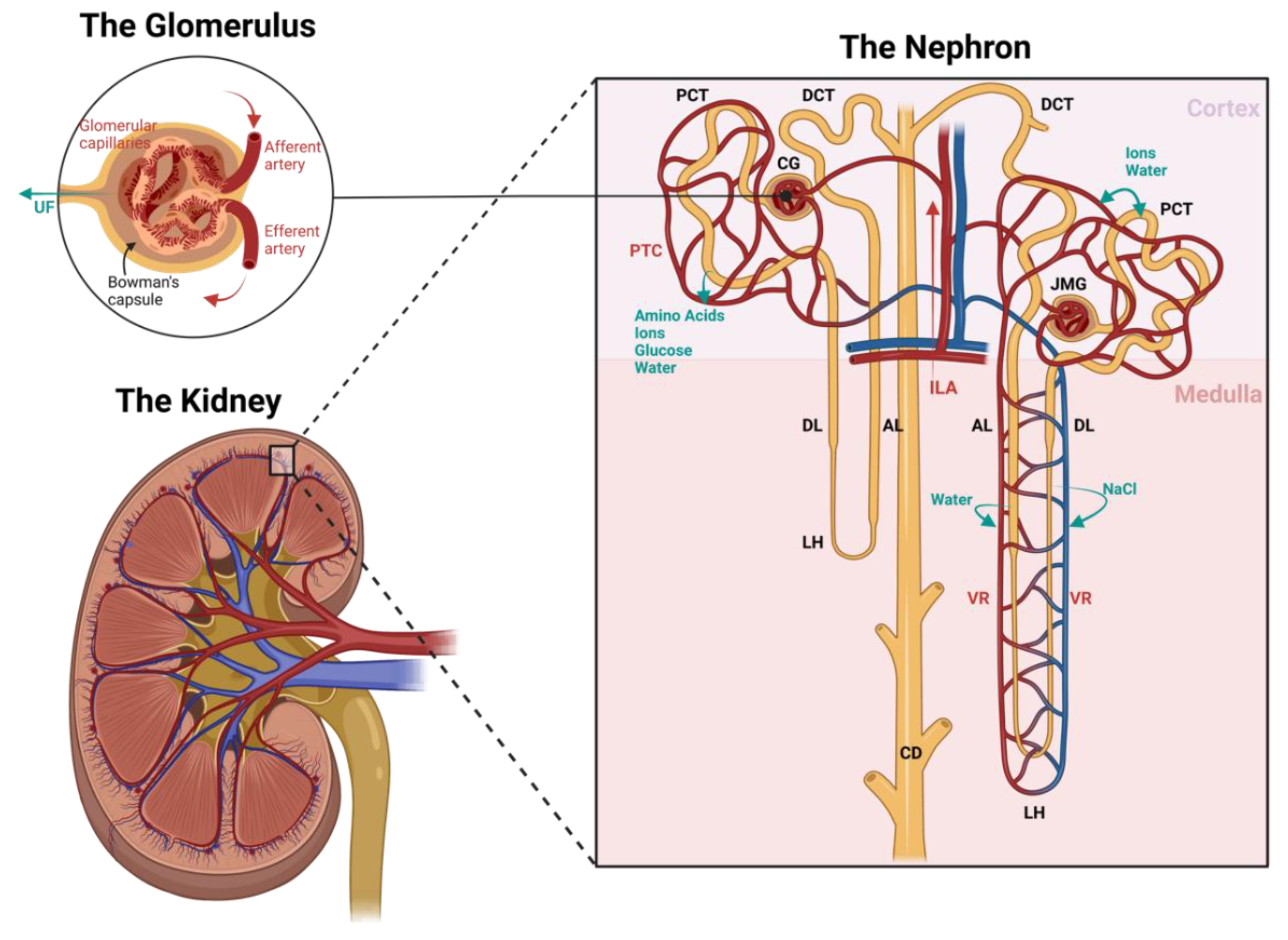
2. Renal Structure and Homeostasis
2.1. Vascular Structure and Function of the Kidney
2.1.1. The Nephron
2.1.2. The Vasculature
2.2. The Importance of Renal Circulation in Homeostasis
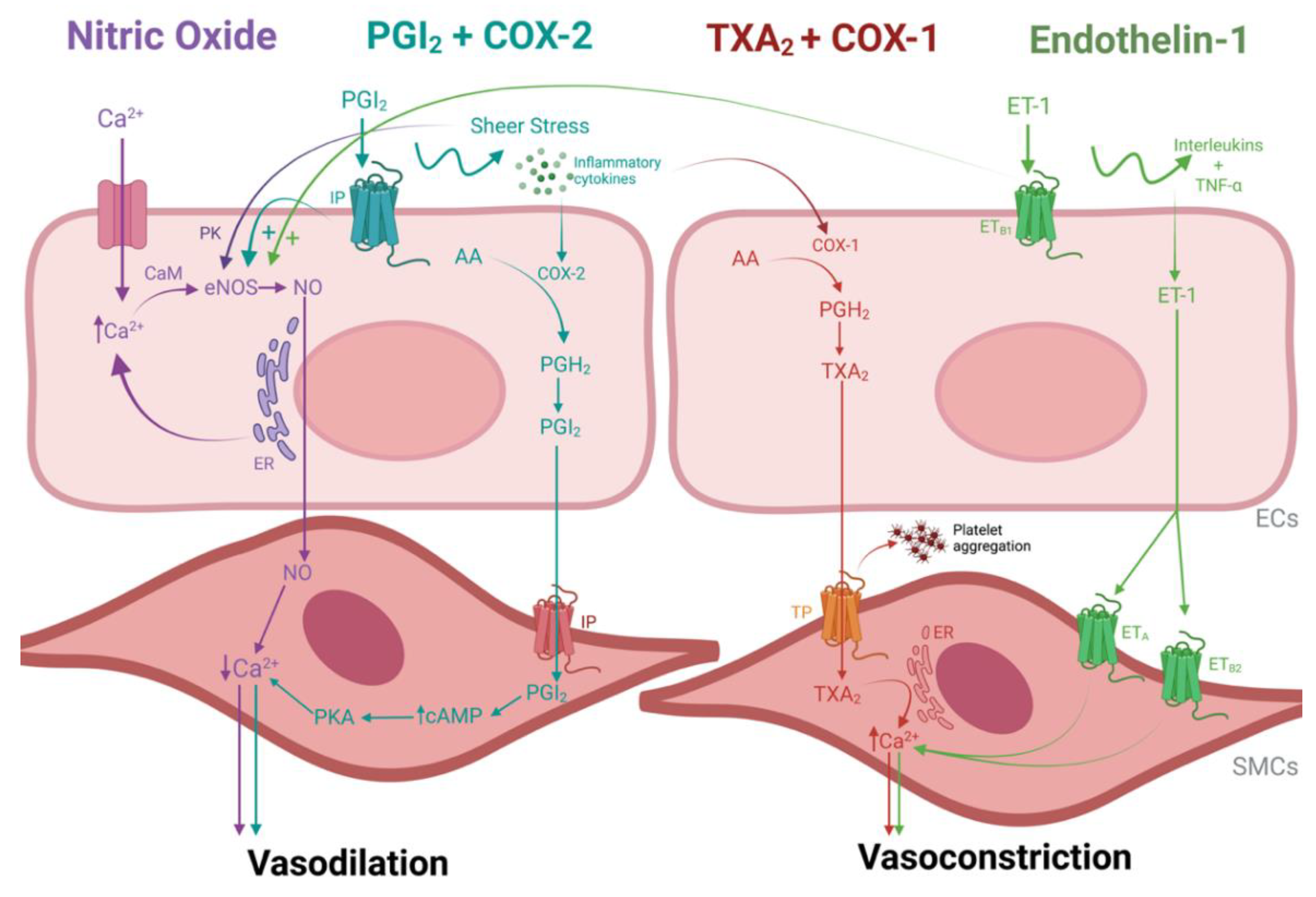
3. Vasoactivity in a Nutshell
3.1. Vasodilation
3.1.1. Nitric Oxide
3.1.2. Prostacyclin and Cyclooxygenase Enzyme 2
3.2. Vasoconstriction
3.2.1. Thromboxane A2 and Cyclooxygenase Enzyme 1
3.2.2. Endothelin-1
4. Vascular (Patho)physiology in the Transplant Setting
4.1. Ischemia-Reperfusion Injury
4.2. Hypoxia Signaling
4.3. Angiogenesis
4.4. Fibrosis
4.5. Coagulation
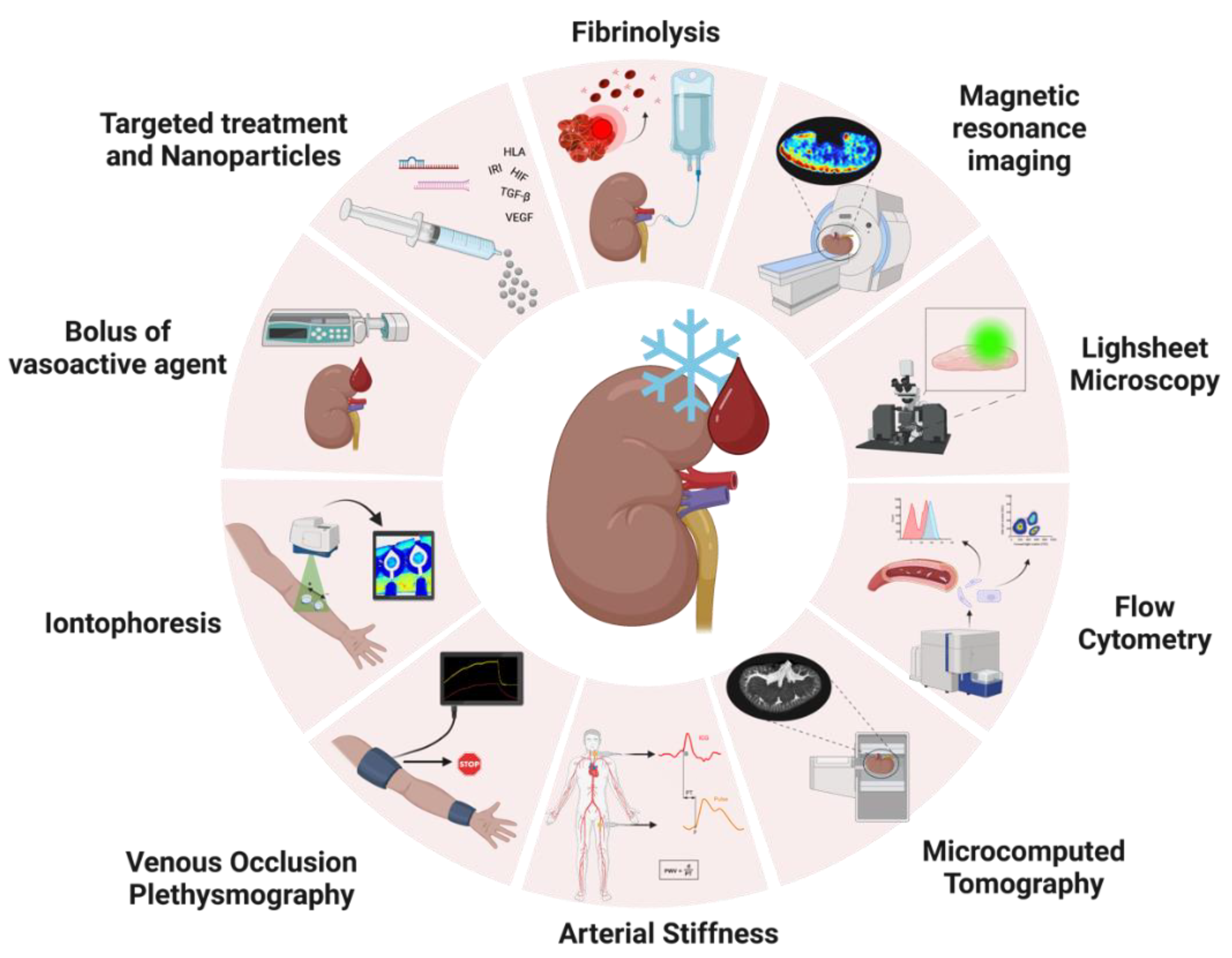
5. Ex Vivo Perfusion Research in a Transplant Setting
5.1. Real-Time Assessment of Vasoactivity during NMP
5.1.1. Endothelial Vasoactive Response Evaluation
5.1.2. Iontophoresis
5.1.3. Venous Occlusion Plethysmography
5.1.4. Arterial Stiffness
5.2. Exploring New Techniques to Unravel Molecular Mechanisms of Vascular Injury
5.2.1. Quantifying Endothelial Cell Shedding with Flow Cytometry
5.2.2. Lightsheet Fluorescence Microscopy
5.2.3. Imaging Renal Microvascular Architecture with Microcomputed Tomography
5.2.4. Visualizing Renal Perfusion Profile with Magnetic Resonance Imaging
5.3. Treating Vascular Injury in an Ex Vivo Setting
5.3.1. Targeting Hypoxia via the HIF Pathways
5.3.2. Targeting Angiogenesis via the VEGF Pathway
5.3.3. Targeting Coagulation with Fibrinolytics
5.3.4. Using siRNA and miRNA to Attenuate Inflammation
5.3.5. Targeting Fibrosis via the TGF-β Pathway
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aimaretti, L.A.; Arze, S. Preemptive Renal Transplantation-The Best Treatment Option for Terminal Chronic Renal Failure. Transplant. Proc. 2016, 48, 609–611. [Google Scholar] [CrossRef]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- De, B.J.; Jochmans, I. Kidney perfusion as an organ quality assessment tool—Are we counting our chickens before they have hatched? J. Clin. Med. 2020, 9, 879. [Google Scholar] [CrossRef]
- Abad, J.B.; Tolosa Eizaguirre, E.; Mayans, A.R.; Rosell Costa, D.; Robles García, J.E.; Zudaire Bergera, J.; Berián Polo, J.M.; Pascual Piedrola, I. Influence of Donor Age on Graft Survival. 2009. Available online: www.elsevier.es/actasuro (accessed on 2 April 2023).
- Snoeijs, M.G.; Schaubel, D.E.; Hené, R.; Hoitsma, A.J.; Idu, M.M.; Ijzermans, J.N.; Ploeg, R.J.; Ringers, J.; Christiaans, M.H.; Buurman, W.A.; et al. Kidneys from Donors after Cardiac Death Provide Survival Benefit. J. Am. Soc. Nephrol. 2010, 21, 1015. [Google Scholar] [CrossRef]
- Heylen, L.; Jochmans, I.; Samuel, U.; Tieken, I.; Naesens, M.; Pirenne, J.; Sprangers, B. The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: A Eurotransplant cohort study. Am. J. Transplant. 2018, 18, 881–889. [Google Scholar] [CrossRef]
- Hamed, M.O.; Chen, Y.; Pasea, L.; Watson, C.J.; Torpey, N.; Bradley, J.A.; Pettigrew, G.; Saeb-Parsy, K. Early graft loss after kidney transplantation: Risk factors and consequences. Am. J. Transplant. 2015, 15, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Srinivasa, S.; Callaghan, C.J.; Watson, C.J.E.; Dark, J.H.; Fisher, A.J.; Wilson, C.H.; Friend, P.J.; Johnson, R.; Forsythe, J.L.; et al. Novel Organ Perfusion and Preservation Strategies in Transplantation-Where Are We Going in the United Kingdom? Transplantation 2020, 104, 1813–1824. [Google Scholar] [CrossRef]
- Hamelink, T.L.; Ogurlu, B.; de Beule, J.; Lantinga, V.A.; Pool, M.B.F.; Venema, L.H.; Leuvenink, H.G.D.; Jochmans, I.M.D.; Moers, C. Renal Normothermic Machine Perfusion: The Road Toward Clinical Implementation of a Promising Pretransplant Organ Assessment Tool. Transplantation 2022, 106, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Akhtar, M.Z.; Nasralla, D.; Kocabayoglu, P.; Boffa, C.; Kaisar, M.; Brat, A.; O’Callaghan, J.; Pengel, L.H.M.; Knight, S. Past, Present, and Future of Dynamic Kidney and Liver Preservation and Resuscitation. Am. J. Transplant. 2016, 16, 2545–2555. [Google Scholar] [CrossRef]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.G.D.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H.G.D. Effects of Oxygen during Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation after Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, S.H.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.; van Heurn, E.; Monbaliu, D.; et al. Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Smith, T.B.; Nicholson, M.L.; Hosgood, S.A. Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation. Transplantology 2021, 2, 460–477. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Van Heurn, E.; Nicholson, M.L. Normothermic machine perfusion of the kidney: Better conditioning and repair? Transpl. Int. 2015, 28, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Hoff, M.; Nicholson, M.L. Treatment of transplant kidneys during machine perfusion. Transpl. Int. 2021, 34, 224–232. [Google Scholar] [CrossRef]
- Bath, M.F.; Hosgood, S.A.; Nicholson, M.L. Vasoreactivity to Acetylcholine During Porcine Kidney Perfusion for the Assessment of Ischemic Injury. J. Surg. Res. 2019, 238, 96–101. [Google Scholar] [CrossRef]
- van Leeuwen, L.L.; Leuvenink, H.G.D.; Olinga, P.; Ruigrok, M.J.R. Shifting Paradigms for Suppressing Fibrosis in Kidney Transplants: Supplementing Perfusion Solutions with Anti-fibrotic Drugs. Front. Med. 2022, 8, 2917. [Google Scholar] [CrossRef] [PubMed]
- DiRito, J.R.; Hosgood, S.A.; Reschke, M.; Albert, C.; Bracaglia, L.G.; Ferdinand, J.R.; Steward, B.J.; Edwards, C.M.; Vaish, A.G.; Thiru, S.; et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am. J. Transplant. 2021, 21, 161–173. [Google Scholar] [CrossRef]
- DiRito, J.R.; Hosgood, S.A.; Tietjen, G.T.; Nicholson, M.L. The future of marginal kidney repair in the context of normothermic machine perfusion. Am. J. Transplant. 2018, 18, 2400–2408. [Google Scholar] [CrossRef]
- Kaths, J.M.; Echeverri, J.; Goldaracena, N.; Louis, K.S.; Chun, Y.; Linares, I.; Wiebe, A.; Foltys, D.B.; Yip, P.M.; John, R.; et al. Eight-hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: A new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation 2016, 100, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Lu, D.B.; Patrick, E.; Xu, B.; Hu, M.; Chew, Y.V.; Keung, K.; P’ng, C.H.; Gaspi, R.; Zhang, C.; et al. Brief Normothermic Machine Perfusion Rejuvenates Discarded Human Kidneys. Transpl. Direct 2019, 5, E502. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Patel, M.; Nicholson, M.L. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J. Surg. Res. 2013, 182, 153–160. [Google Scholar] [CrossRef]
- Bombeli, T.; Karsan, A.; Tait, J.F.; Harlan, J.M. Apoptotic Vascular Endothelial Cells Become Procoagulant. Blood J. Am. Soc. Hematol. 1997, 89, 2429–2442. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.J.; Ferdinand, J.R.; Young, M.D.; Mitchell, T.J.; Loudon, K.W.; Riding, A.M.; Richoz, N.; Fazer, G.L.; Satniforth, J.U.L.; Braga, F.A.V.; et al. Spatio-temporal immune zonation of the human kidney Europe PMC Funders Group. Science 2019, 365, 1461–1466. [Google Scholar] [CrossRef]
- Moore, T.; Ferdinand, J.; Hosgood, S.A.; Clatworthy, M. Normothermic perfusion depletes inflammatory leukocytes in human donor kidneys. Br. J. Surg. 2018, 105, 36. [Google Scholar]
- Adams, T.D.; Hosgood, S.A.; Nicholson, M.L. Physiological effects of altering oxygenation during kidney normothermic machine perfusion. Am. J. Physiol. Ren. Physiol. 2019, 316, 823–829. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Moore, T.; Kleverlaan, T.; Adams, T.; Nicholson, M.L. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J. Transl. Med. 2017, 15, 216. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. An assessment of urinary biomarkers in a series of declined human kidneys measured during ex vivo normothermic kidney perfusion. Transplantation 2017, 101, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, H.; Dieudé, M.; Hébert, M.J. Endothelial dysfunction in kidney transplantation. Front. Immunol. 2018, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Pablo-Moreno, J.A.D.; Serrano, L.J.; Revuelta, L.; Sánchez, M.J.; Liras, A. The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V. Int. J. Mol. Sci. 2022, 23, 8283. [Google Scholar] [CrossRef]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The Role of Endothelium in Physiological and Pathological States: New Data. Biomed. Res. Int. 2018, 2018, 1098039. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G. Biology of the Impaired Endothelium. Am. J. Cardiol. 1991, 68, 6–8. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of Acute Kidney Injury. In Seldin and Giebisch’s the Kidney; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2143–2191. [Google Scholar] [CrossRef]
- Brown, A.L. The Structure of the Nephron. Med. Clin. N. Am. 1966, 50, 927–935. [Google Scholar] [CrossRef]
- Madrazo-Ibarra, A.; Vaitla, P. Histology, Nephron; (Last Update: 17 February 2023); StatPearls: Tampa, FL, USA, 2023. [Google Scholar]
- Kumar, S.; Molitoris, B.A. Renal Endothelial Injury and Microvascular Dysfunction in Acute Kidney Injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef]
- Dumas, S.J.; Meta, E.; Borri, M.; Luo, Y.; Li, X.; Rabelink, T.J.; Carmeliet, P. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat. Rev. Nephrol. 2021, 17, 441–464. [Google Scholar] [CrossRef]
- Kida, Y.; Duffield, J.S. Frontiers in Research: Chronic Kidney Diseases: The pivotal role of pericytes in kidney fibrosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 417. [Google Scholar] [CrossRef]
- Pallone, T.L.; Silldorff, E.P. Pericyte regulation of renal medullary blood flow. Exp. Nephrol. 2001, 9, 165–170. [Google Scholar] [CrossRef]
- Kennedy-Lydon, T.M.; Crawford, C.; Wildman, S.S.P.; Peppiatt-Wildman, C.M. Renal pericytes: Regulators of medullary blood flow. Acta Physiol. 2013, 207, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Ferland-McCollough, D.; Slater, S.; Richard, J.; Reni, C.; Mangialardi, G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol. Ther. 2017, 171, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Rehman, J. Hypoxia signaling in vascular homeostasis. Physiology 2018, 33, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta Biomembr. 2008, 1778, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Veldhuijzen Van Zanten, J.J.C.S.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302. [Google Scholar] [CrossRef]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The role of endothelial glycocalyx in health and disease. Clin. Kidney J. 2019, 12, 611–619. [Google Scholar] [CrossRef]
- Schött, U.; Solomon, C.; Fries, D.; Bentzer, P. The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 48. [Google Scholar] [CrossRef]
- Boo, Y.C.; Sorescu, G.; Boyd, N.; Shiojima, I.; Walsh, K.; Du, J.; Jo, H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser 1179 by Akt-independent mechanisms. Role of protein kinase A. J. Biol. Chem. 2002, 277, 3388–3396. [Google Scholar] [CrossRef]
- Mangana, C.; Lorigo, M.; Cairrao, E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics 2021, 1, 231–251. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.J.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef]
- Cao, Y.; Guan, Y.; Xu, Y.Y.; Hao, C.M. Endothelial prostacyclin protects the kidney from ischemia-reperfusion injury. Pflügers Arch. Eur. J. Physiol. 2019, 471, 543–555. [Google Scholar] [CrossRef]
- Averna, M.; Barbagallo, C.M.; Ganci, A.; Giammarresi, C.; Cefalù, A.B.; Sparacino, V.; Caputo, F.; Basili, S.; Notarbartolo, A.; Davì, G. Determinants of enhanced thromboxane biosynthesis in renal transplantation. Kidney Int. 2001, 59, 1574–1579. [Google Scholar] [CrossRef]
- Chareandee, C.; Herman, W.H.; Hricik, D.E.; Simonson, M.S. Elevated endothelin-1 in tubular epithelium is associated with renal allograft rejection. Am. J. Kidney Dis. 2000, 36, 541–549. [Google Scholar] [CrossRef]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the role of endothelin-1 in atherosclerosis: A potential therapeutic target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Unoki, H.; Iwasa, S.; Watanabe, T. Role of Endothelin-1 in Atherosclerosisa. Ann. NY Acad. Sci. 2006, 902, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, W.H.; Leuvenink, H.G.; Ploeg, R.J. Brain death induced renal injury. Curr. Opin. Organ. Transplant. 2011, 16, 151–156. [Google Scholar] [CrossRef]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.; Steichen, C.; Couturier, P.; Tillet, S.; Mallet, V.; Coudroy, R.; Goujon, J.; Hannaert, P.; Hauet, T. Influence of hypoxic preservation temperature on endothelial cells and kidney integrity. Biomed. Res. Int. 2019, 2019, 8572138. [Google Scholar] [CrossRef]
- Liang, X.; Potenza, D.M.; Brenna, A.; Ma, Y.; Ren, Z.; Cheng, X.; Ming, X.; Yang, Z. Hypoxia Induces Renal Epithelial Injury and Activates Fibrotic Signaling Through Up-Regulation of Arginase-II. Front. Physiol. 2021, 12, 773719. [Google Scholar] [CrossRef]
- Hollis, A.; Patel, K.; Smith, T.; Nath, J.; Tennant, D. Manipulating the HIF Pathway in Renal Transplantation, Current Progress and Future Developments. J. Clin. Exp. Transplant. 2016, 1, 110. [Google Scholar] [CrossRef]
- Tanaka, T.; Nangaku, M. Angiogenesis and hypoxia in the kidney. Nat. Rev. Nephrol. 2013, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Wada, J.; Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 2020, 16, 289–303. [Google Scholar] [CrossRef]
- Doi, K.; Noiri, E.; Fujita, T. Role of Vascular Endothelial Growth Factor in Kidney Disease. Curr. Vasc. Pharmacol. 2010, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.C.; Friedrich, J.L.; Basile, D.P. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F1648–F1657. [Google Scholar] [CrossRef]
- Hara, A.; Wada, T.; Furuichi, K.; Sakai, N.; Kawachi, H.; Shimizu, F.; Shibuya, M.; Matsushima, K.; Yokoyama, H.; Egashira, K.; et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 2006, 69, 1986–1995. [Google Scholar] [CrossRef]
- Shimizu, T.; Nettesheim, P.; Mahler, J.F.; Randell, S.H. Cell type-specific lectin staining of the tracheobronchial epithelium of the rat: Quantitative studies with Griffonia simplicifolia I isolectin B4. J. Histochem. Cytochem. 1991, Published online. [Google Scholar] [CrossRef] [PubMed]
- Sis, B.; Jhangri, G.S.; Bunnag, S.; Allanach, K.; Kaplan, B.; Halloran, P.F. Endothelial gene expression in kidney transplants with alloantibody indicates Antibody-mediated damage despite lack of C4d staining. Am. J. Transplant. 2009, 9, 2312–2323. [Google Scholar] [CrossRef]
- Huwiler, A.; Pfeilschifter, J. Sphingolipid signaling in renal fibrosis. Matrix Biol. 2018, 68, 230–247. [Google Scholar] [CrossRef]
- Huwiler, A.; Pfeilschifter, J. Recuperation of Vascular Homeostasis. Circ. Res. 2021, 129, 237–239. [Google Scholar] [CrossRef]
- Akhter, M.Z.; Chandra Joshi, J.; Balaji Ragunathrao, V.A.; Maieschein-Cline, M.; Proia, R.L.; Malik, A.B.; Mehta, D. Programming to S1PR1 + Endothelial Cells Promotes Restoration of Vascular Integrity. Circ. Res. 2021, 129, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Ávila, A.; Gavela, E.; Sancho, A. Thrombotic Microangiopathy After Kidney Transplantation: An Underdiagnosed and Potentially Reversible Entity. Front. Med. 2021, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, Z. Endothelial Markers: Thrombomodulin and Von Willebrand Factor and Risk of Kidney Thrombosis After Transplantation. Transplant. Proc. 2021, 53, 1562–1569. [Google Scholar] [CrossRef]
- Heeman, W.; Maassen, H.; Calon, J.; Van Goor, H.; Leuvenink, H.G.D.; van Dam, G.M.; Boerma, E.C. Real-time visualization of renal microperfusion using laser speckle contrast imaging. J. Biomed. Opt. 2021, 26, 056004. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R.; Sengar, S. A simple user-made iontophoresis device for palmoplantar hyperhidrosis. J. Cutan. Aesthet. Surg. 2016, 9, 32. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Webb, D.J. Venous occlusion plethysmography in cardiovascular research: Methodology and clinical applications. Br. J. Clin. Pharmacol. 2001, 52, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Scandale, G.; Dimitrov, G.; Recchia, M.; Carzaniga, G.; Minola, M.; Perilli, E.; Carotta, M.; Catalano, M. Arterial stiffness and subendocardial viability ratio in patients with peripheral arterial disease. J. Clin. Hypertens. 2018, 20, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okayama, H.; Nishimura, K.; Ogimoto, A.; Ohtsuka, T.; Inoue, K.; Hiasa, G.; Sumimoto, T.; Higaki, J. Possible link between large artery stiffness and coronary flow velocity reserve. Heart 2008, 94, e20. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; Hall, I.R.; MacCallum, H.; Mackenzie, I.S.; McEniery, C.; van der Arend, B.J.; Shu, Y.; MacKay, L.S.; Webb, D.J.; Cockcroft, J.R. Pulse-wave analysis: Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 147–152. [Google Scholar] [CrossRef]
- Post, I.C.J.H.; Weenink, R.P.; Van Wijk, A.C.W.A.; Heger, M.; Böing, A.N.; van Hulst, R.A.; van Gulik, T.M. Characterization and quantification of porcine circulating endothelial cells. Xenotransplantation 2013, 20, 18–26. [Google Scholar] [CrossRef]
- Lammerts, R.G.M.; Lagendijk, L.M.; Tiller, G.; Dam, W.A.; Lancaster, H.L.; Daha, M.R.; Seelen, M.A.; Hepkema, B.G.; Pol, R.A.; Leuvenink, H.G.D. Machine-perfused donor kidneys as a source of human renal endothelial cells. Am. J. Physiol. Ren. Physiol. 2021, 320, F947–F962. [Google Scholar] [CrossRef]
- Grant, D.; Wanner, N.; Frimel, M.; Erzurum, S.; Asosingh, K. Comprehensive phenotyping of endothelial cells using flow cytometry 2: Human. Cytom. Part A 2021, 99, 257–264. [Google Scholar] [CrossRef]
- Grant, D.; Wanner, N.; Frimel, M.; Erzurum, S.; Asosingh, K. Comprehensive phenotyping of endothelial cells using flow cytometry 1: Murine. Cytom. Part A 2021, 99, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.W.; Loftus, A.F.; Dunn, S.E.; Joens, M.S.; Fitzpatrick, J.A.J. Light Sheet Fluorescence Microscopy (LSFM). Curr. Protoc. Cytom. 2015, 71, 394. [Google Scholar] [CrossRef]
- Puelles, V.G.; Combes, A.N.; Bertram, J.F. Clearly imaging and quantifying the kidney in 3D. Kidney Int. 2021, 100, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Bai, L.; Zong, W.; Wang, X.; Bu, Y.; Zheng, J.; Li, J.; Gao, W.; Feng, Z.; Chen, L.; et al. Light-sheet fluorescence imaging charts the gastrula origin of vascular endothelial cells in early zebrafish embryos. Cell. Discov. 2020, 6, 74. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, J.; Langenbacher, A.D.; Baek, K.I.; Lee, J.; Chang, C.; Hsu, J.J.; Kulkarni, R.P.; Belperio, J.; Shi, W.; et al. Multiscale light-sheet for rapid imaging of cardiopulmonary system. JCI Insight 2018, 3, e121396. [Google Scholar] [CrossRef]
- Prahst, C.; Ashrafzadeh, P.; Mead, T.; Figueiredo, A.; Chang, K.; Richardson, D.; Venkaraman, L.; Richads, M.; Russo, A.M.; Harrington, K.; et al. Mouse retinal cell behaviour in space and time using light sheet fluorescence microscopy. eLife 2020, 9, e49779. [Google Scholar] [CrossRef] [PubMed]
- Farber, G.; Parks, M.M.; Lustgarden Guahmich, N.L.; Zhang, Y.; Monette, S.; Blanchard, S.C.; Di Lorenzo, A.; Blobel, C.P. ADAM10 controls the differentiation of the coronary arterial endothelium. Angiogenesis 2019, 22, 237–250. [Google Scholar] [CrossRef]
- Held, M.; Santeramo, I.; Wilm, B.; Murray, P.; Lévy, R. Ex vivo live cell tracking in kidney organoids using light sheet fluorescence microscopy. PLoS ONE 2018, 13, e0199918. [Google Scholar] [CrossRef]
- Kluza, E. Shedding 3D light on endothelial barrier function in arteries. Atherosclerosis 2020, 310, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Merz, S.F.; Korste, S.; Bornemann, L.; Michel, L.; Stock, P.; Squire, A.; Soun, C.; Engel, D.R.; Detzer, J.; Lörchner, H.; et al. Contemporaneous 3D characterization of acute and chronic myocardial I/R injury and response. Nat. Commun. 2019, 10, 2312. [Google Scholar] [CrossRef] [PubMed]
- Apelt, K.; Bijkerk, R.; Lebrin, F.; Rabelink, T.J. Imaging the renal microcirculation in cell therapy. Cells 2021, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, A.; Hasenberg, A.; Ludwig-Portugall, I.; Medyukhina, A.; Männ, L.; Brenzel, A.; Engel, D.R.; Figge, M.T.; Kurts, C.; Gunzer, M. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 2017, 28, 452–459. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.-D.; et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. [Google Scholar] [CrossRef]
- Bentley, M.D.; Rodriguez-Porcel, M.; Lerman, A.; Sarafov, M.H.; Romero, J.C.; Pelaez, L.I.; Grande, J.P.; Ritman, E.L.; Lerman, L.O. Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int. 2002, 61, 1056–1063. [Google Scholar] [CrossRef]
- Schutter, R.; van Varsseveld, O.C.; Lantinga, V.A.; Pool, M.B.F.; Hamelink, T.H.; Potze, J.H.; Leuvenink, H.G.D.; Laustsen, C.; Borra, R.J.H.; Moers, C. Magnetic resonance imaging during warm ex vivo kidney perfusion. Artif. Organs. 2023, 47, 105–116. [Google Scholar] [CrossRef]
- Schutter, R.; Lantinga, V.A.; Hamelink, T.L.; Pool, M.B.F.; van Varsseveld, O.C.; Potze, J.H.; Hillebrands, J.; van den Heuvel, M.C.; Dierckx, R.A.J.O.; Leuvenink, H.G.D.; et al. Magnetic resonance imaging assessment of renal flow distribution patterns during ex vivo normothermic machine perfusion in porcine and human kidneys. Transpl. Int. 2021, 34, 1643–1655. [Google Scholar] [CrossRef]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef]
- Delpech, P.O.; Thuillier, R.; Le Pape, S.; Rossard, L.; Jayle, C.; Billault, C.; Goujon, J.M.; Hauet, T. Effects of warm ischaemia combined with cold preservation on the hypoxia-inducible factor 1α pathway in an experimental renal autotransplantation model. Br. J. Surg. 2014, 101, 1739–1750. [Google Scholar] [CrossRef]
- Zulpaite, R.; Miknevicius, P.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Ex-vivo Kidney Machine Perfusion: Therapeutic Potential. Front. Med. 2021, 8, 808719. [Google Scholar] [CrossRef]
- Olausson, M.; Antony, D.; Travnikova, G.; Johansson, M.; Nayakawde, N.B.; Banerjee, D.; Søfteland, J.M.; Premaratne, G.U. Novel Ex-Vivo Thrombolytic Reconditioning of Kidneys Retrieved 4 to 5 Hours after Circulatory Death. Transplantation 2022, 106, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Rubanyi, G.M. The role of endothelium in cardiovascular homeostasis and diseases. J. Cardiovasc. Pharmacol. 1993, 22, S1-14. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Qin, L.; Zhang, J.; Abrahimi, P.; Li, H.; Li, G.; Tietjen, G.T.; Tellides, G.; Pober, J.S.; Saltzman, W.M. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat. Commun. 2017, 8, 191. [Google Scholar] [CrossRef]
- Simonson, B.; Das, S. MicroRNA Therapeutics: The Next Magic Bullet? Mini Rev. Med. Chem. 2015, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Van Furth, L.A.; Leuvenink, H.G.D.; Seras, L.; de Graaf, I.A.M.; Olinga, P.; van Leeuwen, L.L. Exploring Porcine Precision-Cut Kidney Slices as a Model for Transplant-Related Ischemia-Reperfusion Injury. Transplantology 2022, 3, 139–151. [Google Scholar] [CrossRef]
| Technique | Previous Application | Advantages | Disadvantages |
|---|---|---|---|
| Response to vasoactive stimuli | Cardiovascular research and ex vivo perfusion | Real-time evaluation | Affects the whole organ |
| Iontophoresis | Clinically used | Real-time evaluation; local effect | Requires validation and placement of chambers on the kidney surface |
| Ultrasound/Laser doppler | Clinically used | Already clinically used; Real-time evaluation; | No generalized assessment possible; Microvasculature not visible; Requires placement of probe on kidney surface; |
| Laser speckle imaging | Ex vivo perfusion | Real-time evaluation; Already validated during ex vivo perfusion; microvasculature more visible | Needs a specific dark chamber for scanning; Only superficial visualization is possible |
| Venous occlusion plethysmography | Clinically used | Real-time evaluation; No need to administer drugs | More aggressive; Affects the whole organ by occlusion of outflow |
| Arterial stiffness | Clinically used | Real-time evaluation; No need to administer drugs; Could possibly be measured in the tubing | Requires validation and possible placement of probes on the kidney surface |
| Flow cytometry | Human, murine studies | Specific analysis/labeling of desired cells/proteins | Not a real-time assessment; Antibody availability could be difficult; |
| Lightsheet microscopy | Murine, zebrafish, organoid studies, etc. | Three-dimensional analysis of sample; Specific analysis/labeling of desired cells/proteins | Not a real-time assessment; Not possible to do whole organ analysis in large animal/human models; Sample needs to be fixed and cleared; Lengthy |
| Microcomputed tomography | Murine in vivo and ex vivo | Three-dimensional analysis of sample; Possible to visualize small structures; Not lengthy; In vivo scanning in small animal models | Not a real-time assessment; Not possible to perform functional analysis |
| Magnetic resonance imaging | Clinically used and ex vivo perfusion | Possible to perform anatomical and functional analysis; Already applied to ex vivo perfusion; Real-time assessment | Still under study for interpretation during ex vivo perfusion; Logistically cumbersome |
| Targeted treatment and nanoparticles | Human, porcine, and murine studies, ex vivo perfusion | Treatment is delivered to the isolated organ; Nanoparticles allow long-term drug delivery | No long-term assessment is possible during ex vivo perfusion; Drug dosage needs validation and tested for toxicity |
| siRNA and/or miRNA treatment | Cardiovascular research, murine and human studies, and ex vivo perfusion | Treatment is delivered to the isolated organ; Treatment can have an effect even after transplantation | No long-term assessment possible during ex vivo perfusion; Drug dosage needs validation and tested for toxicity |
| Precision-cut slices | Murine, porcine, and human studies, ex vivo perfusion studies | Long-term assessment possible; Multiple drugs can be tested in a single organ | Full organ functionality not possible; Prone to infections |
| Fibrinolysis | Human and porcine transplant studies, ex vivo perfusion | Treatment is delivered to the isolated organ; Avoids further graft damage | Post-transplant consequences need more extensive study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos Pamplona, C.; Moers, C.; Leuvenink, H.G.D.; van Leeuwen, L.L. Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion. Curr. Issues Mol. Biol. 2023, 45, 5437-5459. https://doi.org/10.3390/cimb45070345
Campos Pamplona C, Moers C, Leuvenink HGD, van Leeuwen LL. Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion. Current Issues in Molecular Biology. 2023; 45(7):5437-5459. https://doi.org/10.3390/cimb45070345
Chicago/Turabian StyleCampos Pamplona, Carolina, Cyril Moers, Henri G. D. Leuvenink, and L. Leonie van Leeuwen. 2023. "Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion" Current Issues in Molecular Biology 45, no. 7: 5437-5459. https://doi.org/10.3390/cimb45070345
APA StyleCampos Pamplona, C., Moers, C., Leuvenink, H. G. D., & van Leeuwen, L. L. (2023). Expanding the Horizons of Pre-Transplant Renal Vascular Assessment Using Ex Vivo Perfusion. Current Issues in Molecular Biology, 45(7), 5437-5459. https://doi.org/10.3390/cimb45070345







