Abstract
Considerable disturbances in post-translational protein phosphorylation have recently been discovered in multiple neurological disorders. Casein kinase-2 (CK2) is a tetrameric Ser/Thr protein kinase that phosphorylates a large number of substrates and contributes in several cellular physiological and pathological processes. CK2 is highly expressed in the mammalian brain and catalyzes the phosphorylation of a large number of substrates that are crucial in neuronal or glial homeostasis and inflammatory signaling processes across synapses. In this study, we investigated the impact of auditory integration therapy (AIT) for the treatment of sensory processing abnormalities in autism on plasma CK2 levels. A total of 25 ASD children, aged between 5 and 12 years, were enrolled and participated in the present research study. AIT was performed for two weeks, for a period of 30 min, twice a day, with a 3 h interval between sessions. Before and after AIT, the Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS), and Short Sensory Profile (SSP) scores were calculated, and plasma CK2 levels were assayed using an ELISA test. The CARS and SRS indices of autism severity improved as a result of AIT, which could be related to the decreased level of plasma CK2. However, the mean value of the SSP scores was not significantly increased after AIT. The relationship between CK2 downregulation and glutamate excitotoxicity, neuro-inflammation, and leaky gut, as etiological mechanisms in ASD, was proposed and discussed. Further research, conducted on a larger scale and with a longer study duration, are required to assess whether the cognitive improvement in ASD children after AIT is related to the downregulation of CK2.
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder which is characterized by impaired social interaction and communication, stereotypic and restricted behaviors, and abnormal sensory reactivity. Within the last several decades, the incidence rate of ASD has increased dramatically, and cases of ASD have been reported at a rate of 0.6–0.8% in infants, and 1.0% in school-age children and young adults [1,2]. Under this circumstance, it is urgent to understand the mechanisms involved in the development of ASD to enable the early detection of biomarkers that could help improve timely diagnosis, with a significant influence on lifelong prognosis [3].
Most proteins in mammalian cells are phosphorylated as a dynamic post translational modification that can control protein folding, interactions, localization, and stability [4,5,6]. Phosphorylation adds two negative charges to the protein at physiological pH, which will modify the electrostatic milieu and can change the strength of protein–protein interactions [4,6]. Although the estimated stability of most protein complexes is not altered by phosphorylation, about one-third of these complexes is expected to be significantly stabilized or destabilized by phosphorylation.
Casein kinase-2 (CK2) is a tetrameric Ser/Thr protein kinase that phosphorylates a large number of substrates and contributes to numerous cellular physiological and pathological processes, such as proliferation, survival, apoptosis, angiogenesis, endoplasmic reticulum stress response, DNA damage and repair, carbohydrate metabolism, and most importantly, brain development [7]. CK2 is regularly expressed in the periphery, as well as in the brain [8]. However, there is currently insufficient information regarding the role of CK2 in brain development. Lettieri et al. [8] reported that the loss of CK2 α’ and β subunits severely interrupts GN11 neuronal cell line migration, as well affects cell adhesion, through the activation of diverse signaling pathways, thus providing the first proof of CK2 importance in neuron migration. This may be related to the observation that mutations in genes encoding for CK2 subunits have been identified in patients clinically presenting with NDDs, supporting the idea that CK2 is certainly essential for appropriate neuronal migration during brain development.
CK2 has been identified in the plasma membrane, as well as in the nucleus and cytoplasm of neurons [9], more specifically at the postsynaptic density in rat hippocampus and cortical preparations [10]. CK2 activity is enhanced in synaptosomes [11], and a plethora of CK2 substrates discovered in vitro or in vivo convincingly link CK2 to synaptic activity modulation [12]. CK2 regulates the homeostasis of neurotransmitter receptors such as ion channel receptors and G-proteins coupled receptors (GPCRs) [13,14]. The NMDA glutamate receptor, a cation channel for Ca2+, Na+, and K+, plays important roles in synaptic plasticity, memory, and learning.
Abnormal neuronal migration in individuals with autism, as well as in rodent models, has been detected in most brain regions relevant to autistic behaviors [15]. Among the most common recorded abnormal migration in ASD are the migration of GABAergic interneuron and glutamatergic neuron cell types [16,17]. This can be related to the well-documented reduced GABAergic inhibitory tone which occurs in autistic brains [18], resulting in the altered excitation/inhibition (E/I) balance of ASD-relevant brain circuits [18,19].
In relation to ASD, it is interesting to note that CK2 interacts with the autism susceptibility candidate 2 (AUTS2) gene as an emerged crucial gene associated with a wide range of neurodevelopmental disorders, among which is ASD [20,21]. Okur et al. [22] postulate that the mutations that alter CK2 function and the phosphorylation of CK2 targets lead to deleterious effects on brain development and function. It has been proposed that CK2 is a cause of syndromic developmental delay, and perhaps autism spectrum disorder [23].
Furthermore, CK2 has been proposed as the primary α-synuclein Ser129 kinase in the brain. This could support the link between CK2 levels in ASD individuals and the severity of the disease. Alpha-synuclein phosphorylation was first proposed as a factor in protein toxicity and aggregation formation. Surprisingly, in subjects with neurological diseases, almost 90% of α-synuclein Ser129 sites are phosphorylated, compared to only 4% or less in subjects without these diseases. CK2 inhibition appears to be advantageous in a variety of neurological conditions, including ASD, according to various studies [7,24]. Increased plasma levels of α-synuclein have recently been reported in people with ASD and have been linked to the development and severity of the illness [25,26,27,28].
Intestinal permeability reveals the sum of the functionally discrete tight junction pore and the leakage pathways. The tight junction pore pathway is a high capacity, size- and charge-selective passageway whose permeability is mostly controlled by a subset of claudin family proteins [29,30]. Interestingly, it was found that the inhibition of CK2 blocks claudin-2 channel function through the prevention of IL-13-induced claudin-2 upregulation, increasing gut permeability in vivo [31]. Moreover, Dörfel et al. [32] reported that CK2-dependent occludin phosphorylation impaired its binding with zonulin-2 protein, greatly affecting tight junction integrity, and thus increasing intestinal permeability.
Although the most effective approach to intervention is not clear, the scenario of ASD patients can be improved with early intervention [33]. Auditory integrative training (AIT) was developed to enhance aberrant sound sensitivity, especially for behavioral disorders such as ASD [34]. AIT may be helpful as an intervention technique for people with ASD, according to Rotschafer et al. [35], due to the fact that anomalous sensitivity or insensitivity to specific sound wave frequencies, regardless of overall hearing capacity, is related to some form of behavior and learning difficulties.
Considering the crucial role of CK2 in the development of the brain [14], it is of great interest to study the role of CK2 in the etiology of autism. Therefore, the purpose of this study was to investigate potential AIT effects on plasma CK2 levels, as well as the relationship between these levels and the improvement of social and cognitive impairment in AIT-trained individuals.
2. Materials and Methods
The Autism Research and Treatment Center at King Khalid University Hospital in Riyadh, King Saud University, and the Kingdom of Saudi Arabia were the sources of participants for this study. The study included 25 male ASD patients, with ages ranging from 5 to 12 years. Utilizing the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), all individuals were tested and assessed. The CARS, SRS, and SSP scores were computed prior to and following intervention (i.e., immediately after, one month, and three months after AIT for each child). AIT sessions were conducted twice daily for two weeks, with each session lasting 30 min, and 3 h breaks in between sessions. The study excluded children with a history of seizures. The schematic design of the study is summarized in Scheme 1.
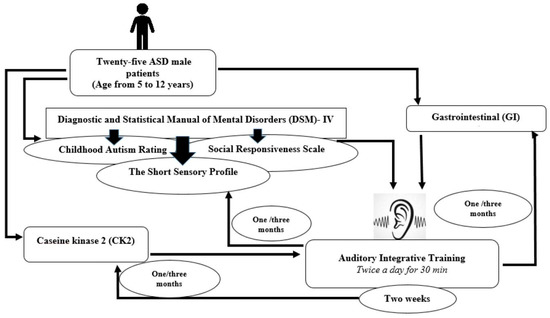
Scheme 1.
A schematic design of the study.
Written consent was obtained from the parents of each patient, according to the guidelines of the Ethics Committee of the King Saud University, King Khalid University Hospital. Children were not permitted to start new treatments, or terminate existing treatments, including the use of prescription drugs and dietary supplements, during the AIT intervention period. The Institutional Review Board of the College of Medicine at King Saud University granted ethical approval for the study.
2.1. Childhood Autism Rating Scale
The CARS score was evaluated as a measurement of the severity of autism. The child is assessed by using a scale of 1 to 4 for each of 15 characteristics or symptoms, including verbal communication; listening response; fear or nervousness; imitation; body use; object use; ability to relate to people; emotional response; nonverbal communication; activity level; level and reliability of intellectual response; adaptation to changes; visual response; taste, smell, and touch responses; and general impressions. The presence of autism is strongly suggested by a total score of at least 30. While children with scores between 37 and 60 have severe autism, those between 30 and 36 have mild-to-moderate autism [36].
2.2. Social Responsiveness Scale
The SRS is an authorized test of social behavior, stereotypical traits, and communication in autism [36]. It is used as a diagnostic instrument, differentiating clinically presenting ASD children from others with different levels of social interaction impairment, but who exhibit non-ASD psychiatric disorders. It consists of 5 subscales: (1) social awareness, (2) social cognition, (3) social communication, (4) social motivation, and (5) autistic mannerisms. Total SRS scores range from 0 to 195, following or evaluating the significant social impairment as observed in individuals with ASD. A score between 60 and 75 is in the mild-to-moderate range of social impairment, while a score of 76 or higher is considered severe and is strongly associated with a clinical diagnosis of ASD [37].
2.3. The Short Sensory Profile
The 38-item SSP questionnaire, which is intended for kids aged 3 to 14, offers brief details regarding the sensory-processing abilities of autistic kids [38]. Each SSP item is scored on a 5-point Likert scale. Domain scores for the areas of movement sensitivity, wanting sensation, auditory filtering, low energy levels, and visual/auditory sensitivity were measured. The categories of typical performance, probable variation from usual performance, and definitely different from typical performance were used to evaluate domain scores and overall sensory responses. Scores between 143 and 152 indicate mild-to-moderate performance (probably different from typical performance), scores between 153 and 190 show typical performance, and scores below 142 represent severely different performance (clear deviation from typical performance). Numerous studies have employed the SSP [39]. The Auditory Integration Training AIT was conducted in accordance with a published technique previously used by our team [40].
Participants were initially examined by a medical doctor to confirm that there was no wax and/or fluid in their ears. They then participated in 20–30-min hearing sessions for a 15- to 20-day period, with a break of 1 or 2 days after 5 therapy days. The child listened to recorded music during these sessions. The AIT sound amplifier attenuated low and high frequencies from the CDs at random before sending the changed music to the listener through headphones. Depending on the person’s comfort level, the volume during the AIT sessions was set at much lower intensities and did not go above 80 dBA (low scale). The volume of the music was generally considered to be safe.
2.4. Measurement of Plasma Casein Kinase-2 (CK2)
Fasting blood samples from each child were collected in test tubes containing EDTA, the samples were immediately centrifuged at 3000 rpm, and plasma was collected and stored at −80 °C until analysis. All samples were assayed in duplicate and in a double-blind manner. CK2 concentrations were measured in the plasma of autistic subjects using a commercially available sandwich ELISA kit, a product of Cusabio Biotech Co. Ltd., Wuhan, China. Plasma CK2 levels were measured before AIT and again immediately after, one month after, and three months after AIT, according to the manufacture instructions. To improve accuracy, all samples in the current investigation were tested in two independent trials as duplicates to confirm repeatability and detect inter-assay variances in the results (p < 0.05). No significant interference or cross-reactivity was identified.
2.5. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS 21.0 for Windows; SPSS, Chicago, IL, USA) was used to analyze the data. Mean SD was used to express results. Using repeated-measure analysis of variance, significant measured changes in the parameters were evaluated. Significant differences were also evaluated using Bonferroni multiple comparison tests.
The receiver operating characteristic (ROC) analysis approach was used to evaluate the prognostic and predictive value of CK2 after AIT treatment in relation to CARS, SRS, and SP as three measures of ASD severity. ROC is common statistical tool used for evaluating the diagnostic validity of biomarkers commonly used in clinical psychology. To accomplish this evaluation, a cut-off point must be set. The area under the ROC curve (AUROC) can be used to estimate how well a diagnostic variable is performing. A random prediction would have an AUROC of 0.5, while the perfect test would have an AUROC of 1.
3. Results and Discussion
The changes in CK2 levels and the three behavioral rating scales (CARS, SRS, and SSP) before, immediately after, one month after, and three months after AIT are listed as means ± SD in Table 1, Table 2, Table 3 and Table 4. The plasma levels of CK2 significantly reduced by 18.92% immediately after AIT (p < 0.049), by 14.02 one month after AIT (p < 0.052), and by 16.96% three months after AIT (p < 0.046) compared to the results before AIT intervention (Table 1 and Figure 1). Scores of CARS, an indicator of autism severity, were decreased by 17% one month after AIT (p < 0.05) compared to before AIT (Table 2 and Figure 2). The total SRS scores significantly decreased (20.36%), and the total SSP scores were non-significantly increased three months after AIT (p < 0.612) (Table 3 and Figure 3). SP was non-significantly increase post AIT training (Table 4 and Figure 4). Table 5 demonstrates the remarkable decrease in GI symptoms among the AIT- treated participants. This suggests that for certain kids with ASD, AIT may exhibit a major therapeutic value. Table 6 and Figure 5 show the ROC analysis AUCs, specificity, and sensitivity of CK2 (immediately, one month after, and three months after), as well as SRS, CARS, and SSP (one and three months after). In the ROC analysis, the results can be interpreted as follows: AUC < 0.70, low diagnostic accuracy; AUC in the range of 0.70–0.90, moderate diagnostic accuracy; and AUC ≥ 0.90, high diagnostic accuracy.

Table 1.
Effect of auditory integrative training (AIT) on caseine kinase 2 (CK2) in children with autism (n = 25).

Table 2.
Effect of auditory integrative training (AIT) on CARS scores of children with autism (n = 25).

Table 3.
Effect of auditory integrative training (AIT) on SRS scores of children with autism (n = 25).

Table 4.
Effect of auditory integrative training (AIT) on SP scores of children with autism (n = 25).
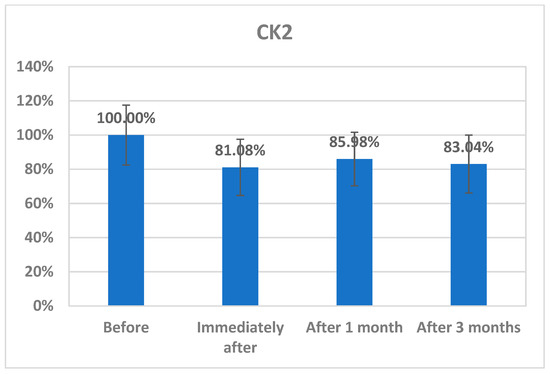
Figure 1.
Percentage change in CK2 concentrations in plasma of autistic patients immediately, one month, and three months post AIT compared to before.
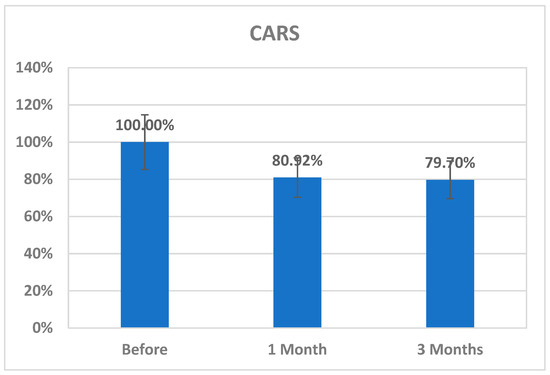
Figure 2.
Percentage change in CARS scores of autistic patients one month and three months post AIT compared to before.
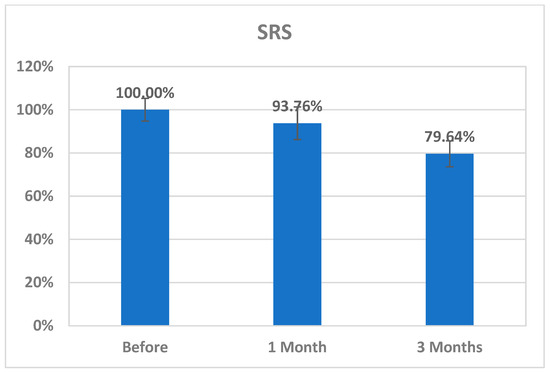
Figure 3.
Percentage change in SRS of autistic patients one month and three months post AIT compared to before.
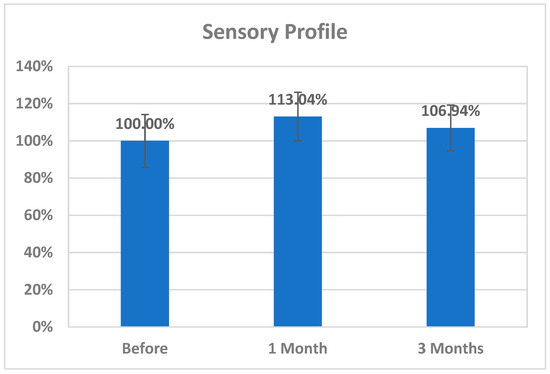
Figure 4.
Percentage change in SP of autistic patients one month and three months post AIT compared to before.

Table 5.
Effect of auditory integrative training (AIT) on gastrointestinal (GI) symptoms before, one month, and three months post training.

Table 6.
ROC curves of CK2, CARS, SRS, and SP in different studied durations.
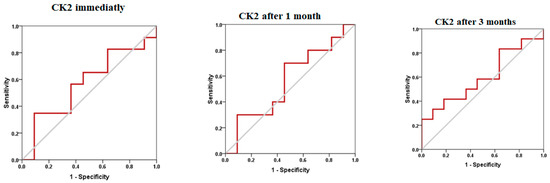
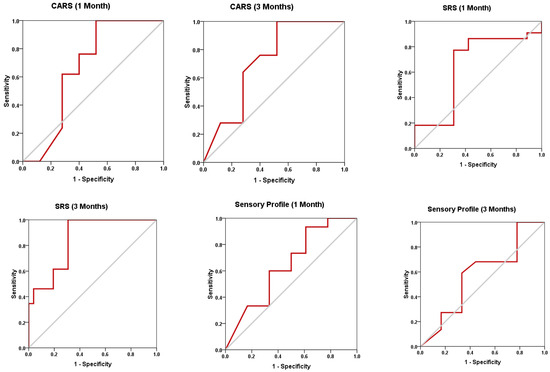
Figure 5.
ROC curves of CK2, CARS, SRS, and SP post AIT intervention.
Table 2 describes paired samples t-test (parametric data) between each period (1 month and 3 months) and before using CARS scores.
Table 3 describes paired samples t-test (parametric data) between each period (1 month and 3 months) and before using SRS scores.
Table 4 describes paired samples t-test (parametric data) between each period (1 month and 3 months) and before using the sensory profile.
In recent years, complementary alternative medicine (CAM) treatments received increased attention from the scientific community: numerous studies have been conducted in order to examine the effectiveness and safety of CAMs in ASD. Unfortunately, there is a lack of evidence regarding the usefulness of CAM in ASD. There is remarkable contrast between the rate of use of CAM by families with autistic children and the lack of scientific outcomes of alternative treatments. One probable cause for this difference is that CAM remedies are generally considered as “natural”, with an optimum safety profile and fewer or the absence of side effects when compared to those of conventional drugs [40].
In the present study, AIT training intervention for three months significantly decreased CK2, with concomitant improvement of CARS, SRS, and SSP as measures of ASD severity, and GI as a co-morbidity of ASD.
Based on our current understanding of the etiology of ASD, many blood-based biomarker candidates have been investigated [41,42], particularly neurotransmitters [42], proinflammatory cytokines [42], markers of mitochondrial dysfunction [41,43], and markers of oxidative stress and impaired gut microbiota [44]. Most recently, Montanari et al. [45] reported that glutamatergic neurotransmission is highly indicated as an etiological mechanism in the pathophysiology of ASD, and it is considered to be directly related to ASD severity, identifying it as a potential target for novel management through which other etiological mechanisms could be also controlled or managed [41,45,46].
Proinflammatory cytokines (e.g., TNFα and IL-1β) negatively regulate glutamate transporter expression and activity, increasing extracellular glutamate concentrations [47]. In turn, immune activation increases cystine–glutamate exchanger (xCT) expression, possibly resulting in higher glutamate release and excitotoxic damage to oligodendrocytes [48,49].
In an attempt to determine the correlation between the reported significant decrease in CK2 (Table 1 and Figure 1) and the remarkable improvements in CARS and SRS scores, as measures of ASD severity previously related to glutamate excitotoxicity (Table 2, Table 3 and Table 4 and Figure 2, Figure 3 and Figure 4), it was of interest to emphasize the role of CK2 in phosphorylating glutamate receptors and/or transporters as protein components of the glutamate signaling pathway and to identify its involvement in pro-inflammation and apoptosis as critical events in ASD [50]. The significant improvement in CARS, SRS, and non-significant increase in SSP scored could be related to the significant reduction of CK2 activity one and three months post AIT. This could be explained on the basis that, in addition to CK2 apoptotic function, a number of studies have suggested its pro-inflammatory role and the possibility that CK2 pharmacological inhibition attenuates apoptosis and neuroinflammation as etiological mechanisms of many diseases, among which is ASD [51,52,53,54]. Moreover Canedo-Antelo et al. [55] recorded that CK2 inhibition rescues cultured oligodendrocytes from AMPA receptors and glutamate induced excitotoxic death.
It is well accepted and proved that both peripheral and brain inflammatory responses are suggested to be associated with ASD-related behavioral symptoms. The suggested association between the AIT-induced CK2 downregulation and the observed improvement of SRS and CARS scores is supported by the work of Hafizi et al. [56], in which treatment with memantine and lenalidomide as anti-inflammatory drugs was associated with significant improvement in SRS and CARS scores. Moreover, the effectiveness of this treatment can be supported by considering the remarkable improvements in communication, daily living skills, social skills, and stereotypical behavior as measured by the Autistic Behavior Checklist (ABC) in response to treatment of inflammation using natural flavonoid luteolin. A positive correlation was recorded between behavioral improvement and the reduction in the serum levels of IL-6 and TNF following treatment with luteolin over a 12-month period [57,58]. The non-significant changes in SSP scores reported in the present study (Table 4 and Figure 4) could be attributed to the fact that their interpretation is complicated by limited content validity and considerable bias due to the multidimensionality of its integral parts. Williams et al. discouraged the use of the SSP total score and most subscale scores in children with ASD [59].
The altered gut flora in ASD has been linked to increased gut permeability, or ”leaky gut”, which allows bacterial metabolites to pass through the gut barrier and affect early childhood neurodevelopment in vulnerable individuals via the gut–brain axis. The studies of Raleigh et al. [60] provided fascinating preliminary insight into the intricate role of CK2 in the creation of TJs. Improvements in the transepithelial electrical resistance (TER) values, as well as a reduction in paracellular Na+-flux, resulted from treating the barrier function of Caco-2 cells with different CK2 inhibitors or the siRNA-mediated knockdown of CK2. Tight junctional occludin production increased when CK2 was suppressed, which might help to treat gut leakiness. Table 5 demonstrates the remarkable decrease in GI symptoms among the AIT-treated participants. Again, this could be related to the lower recorded levels of CK2 in AIT-treated patients. Lower CK2 levels might be accompanied by remarkable improvement in the tight junction integrity and intestinal permeability.
ROC analysis presented in Table 6 and Figure 5 could help to suggest that among the four measured variables, while CK2 and SSP recorded low diagnostic value, with AUCs less than 0.7, CARS and SRS demonstrated moderate diagnostic value (AUCs of 0.7–0.9 range) as measures of the effect of AIT as CAM in ASD patients.
CK2 is considered as a potential therapeutic target due to its involvement in several neurological and psychiatric disorders. The vast range of inhibitors that are already accessible and might currently be in the hands of practitioners could be suggested as an intervention approach in ASD, in addition to AIT therapy [61].
Scheme 2 demonstrates the effect of AIT on CK2, CARS, SRS, and SSP scores, as well as GI as a co-morbidity of ASD. AIT significantly decreases CK2, yielding improved CARS, SRS, and SSP scores. A significant decrease in CK2 can improve CARS, SRS, and SSP scores in autistic patients through the reduced phosphorylation of glutamate receptors, diminished apoptosis, and decreased neuroinflammation. Tight junction integrity and intestinal permeability, which are required for a healthy gut, are improved by suppressing CK2 activity. The inhibition of CK2 can improve GI health in autistic patients via enhanced transepithelial electrical resistance and tight junctional occluding, with reduced paracellular Na+-flux.
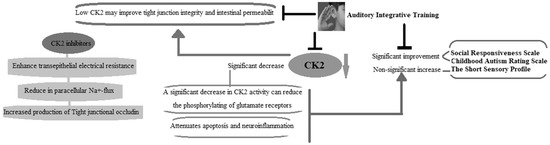
Scheme 2.
Illustrates how AIT affects scores for CK2, CARS, SRS, and SSP, in addition to GI as a co-morbidity of ASD.
4. Conclusions
The findings reported here indicate the effectiveness of AIT as a tool of CAM as an intervention strategy in the treatment of ASD. Lower CK2 in AIT-treated participants, together with the significant improvement in SRS and CARS scores, indicate the possible ameliorative effects of neuroinflammation, apoptosis, excitotoxic, and leaky gut conditions as pathological mechanisms in autism. We propose that CK2 inhibition could be used as a promising target to lessen the severity of ASD.
Author Contributions
L.A.-A.: conceptualization, AIT intervention, and follow-up post AIT evaluation; R.S.B., F.A.A. and A.S.A.: data acquisition; A.E.-A.: conceptualization and drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Plan for Science Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (award number: 08-MED 510–02).
Institutional Review Board Statement
This work was approved by the Ethics Committee of the King Saud University King Khalid Hospital.
Informed Consent Statement
Written consent was obtained from the parents of each subject, according to the guidelines of the Ethics Committee of the King Saud University King Khalid Hospital.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Dowling, N.F. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1. [Google Scholar] [CrossRef] [PubMed]
- Brugha, T.S.; Spiers, N.; Bankart, J.; Cooper, S.A.; McManus, S.; Scott, F.J.; Smith, J.; Tyrer, F. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry 2016, 209, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Fernell, E.; Landgren, M.; Lindström, K.; Johnson, M.; Gillberg, C. Children and young people with neurodevelopmental problems: Support and efforts must be given even if not all diagnostic criteria are met. Lakartidningen 2013, 110, 1674. [Google Scholar] [PubMed]
- Narayanan, A.; Jacobson, M.P. Computational studies of protein regulation by post-translational phosphorylation. Curr. Opin. Struct. Biol. 2009, 19, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative phosphoproteomics revealswidespread full phosphorylation site occupancy during mitosis. Sci. Signal. 2010, 3, ra3. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Nonaka, T.; Taniguchi, S.; Saito, T.; Arai, T.; Mann, D.; Iwatsubo, T.; Hisanaga, S.-I.; Goedert, M.; Hasegawa, M. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human α-synuclein: Implication for α-synucleinopathies. FEBS Lett. 2007, 581, 4711–4717. [Google Scholar] [CrossRef]
- Götz, C.; Montenarh, M. Protein kinase CK2 in development and differentiation (Review). Biomed. Rep. 2017, 6, 127–133. [Google Scholar] [CrossRef]
- Lettieri, A.; Borgo, C.; Zanieri, L.; D’amore, C.; Oleari, R.; Paganoni, A.; Pinna, L.A.; Cariboni, A.; Salvi, M. Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons. Int. J. Mol. Sci. 2019, 20, 5951. [Google Scholar] [CrossRef]
- Rebholz, H.; Nishi, A.; Liebscher, S.; Nairn, A.C.; Flajolet, M.; Greengard, P. CK2 Negatively Regulates G S Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 14096–14101. [Google Scholar] [CrossRef]
- Soto, D.; Pancetti, F.; Marengo, J.J.; Sandoval, M.; Sandoval, R.; Orrego, F.; Wyneken, U. Protein Kinase CK2 in Postsynaptic Densities: Phosphorylation of PSD-95/SAP90 and NMDA Receptor Regulation. Biochem. Biophys. Res. Commun. 2004, 322, 542–550. [Google Scholar] [CrossRef]
- Girault, J.A.; Hemmings, H.C., Jr.; Zorn, S.H.; Gustafson, E.L.; Greengard, P. Characterization in mammalian brain of a DARPP-32 serine kinase identical to casein kinase II. J. Neurochem. 1990, 55, 1772–1783. [Google Scholar] [CrossRef]
- Castello, J.; Ragnauth, A.; Friedman, E.; Rebholz, H. CK2—An emerging target for neurological and psychiatric disorders. Pharmaceuticals 2017, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, M.; Götz, C. Protein Kinase CK2 and Ion Channels (Review). Biomed. Rep. 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Castello, J.; LeFrancois, B.; Flajolet, M.; Greengard, P.; Friedman, E.; Rebholz, H. CK2 Regulates 5-HT4 Receptor Signaling and Modulates Depressive-like Behavior. Mol. Psychiatry 2018, 23, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Yanagi, S. Psychiatric behaviors associated with cytoskeletal defects in radial neuronal migration. Cell. Mol. Life Sci. 2017, 74, 3533–3552. [Google Scholar] [CrossRef]
- Wegiel, J.; Kuchna, I.; Nowicki, K.; Imaki, H.; Wegiel, J.; Marchi, E.; Ma, S.Y.; Chauhan, A.; Chauhan, V.; Bobrowicz, T.W.; et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010, 119, 755–770. [Google Scholar] [CrossRef]
- Provenzano, G.; Chelini, G.; Bozzi, Y. Genetic control of social behavior: Lessons from mutant mice. Behav. Brain Res. 2017, 325, 237–250. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.J. Pv interneurons: Critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism–epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef]
- Gao, Z.; Lee, P.; Stafford, J.M.; Von Schimmelmann, M.; Schaefer, A.; Reinberg, D. An AUTS2–Polycomb complex activates gene expression in the CNS. Nature 2014, 516, 349–354. [Google Scholar] [CrossRef]
- Hori, K.; Shimaoka, K.; Hoshino, M. AUTS2 Gene: Keys to Understanding the Pathogenesis of Neurodevelopmental Disorders. Cells 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Okur, V.; Cho, M.T.; Henderson, L.; Retterer, K.; Schneider, M.; Sattler, S.; Niyazov, D.; Azage, M.; Smith, S.; Picker, J.; et al. De novo mutations in CSNK2A1 are associated with neurodevelopmental abnormalities and dysmorphic features. Hum. Genet. 2016, 135, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Gold, N.B.; Li, D.; Chassevent, A.; Kaiser, F.J.; Parenti, I.; Strom, T.M.; Ramos, F.J.; Puisac, B.; Pié, J.; McWalter, K.; et al. Heterozygous de novo variants in CSNK1G1 are associated with syndromic developmental delay and autism spectrum disorder. Clin. Genet. 2020, 98, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Walter, J.; Koyama, A.; Nakajo, S.; Baba, M.; Iwatsubo, T.; Meijer, L.; Kahle, P.J.; Haass, C. Constitutive Phosphorylation of the Parkinson’s Disease Associated α-Synuclein. J. Biol. Chem. 2000, 275, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? J. Park. Dis. 2016, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Al-Mazidi, S.; Al-Ayadhi, L.Y. Plasma Levels of Alpha and Gamma Synucleins in Autism Spectrum Disorder: An Indicator of Severity. Med. Princ. Pract. 2021, 30, 160–167. [Google Scholar] [CrossRef]
- El-Ansary, A.; Alhakbany, M.; Aldbass, A.; Qasem, H.; Al-Mazidi, S.; Bhat, R.S.; Al-Ayadhi, L. Alpha-Synuclein, cyclooxygenase-2 and prostaglandins-EP2 receptors as neuroinflammatory biomarkers of autism spectrum disorders: Use of combined ROC curves to increase their diagnostic values. Lipids Health Dis. 2021, 20, 155. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Ikewaki, N.; Sonoda, T.; Iwasaki, M.; Preethy, S.; Abraham, S.J. Improvement of behavioural pattern and alpha-synuclein levels in autism spectrum disorder after consumption of a beta-glucan food supplement in a randomised, parallel-group pilot clinical study. BMJ Neurol. Open 2022, 4, e000203. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Dörfel, M.J.; Westphal, J.K.; Bellmann, C.; Krug, S.M.; Cording, J.; Mittag, S.; Tauber, R.; Fromm, M.; Blasig, I.E.; Huber, O. CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. Cell Commun. Signal. 2013, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Marie, J.C.; Pelletier, A.L.; Song, Z.; Ben-Khemis, M.; Boudiaf, K.; Pintard, C.; Leger, T.; Terrier, S.; Chevreux, G.; et al. Protein Kinase CK2 Acts as a Molecular Brake to Control NADPH Oxidase 1 Activation and Colon Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Paul, R. Interventions to Improve Communication in Autism. Child Adolesc. Psychiatr. Clin. North Am. 2008, 17, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Sinha, Y.; Silove, N.; Hayen, A.; Williams, K. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2011, 2011, CD003681. [Google Scholar] [CrossRef]
- Rotschafer, S.E. Auditory Discrimination in Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 651209. [Google Scholar] [CrossRef]
- Ventola, P.E.; Kleinman, J.; Pandey, J.; Barton, M.; Allen, S.; Green, J.; Robins, D.; Fein, D. Agreement Among Four Diagnostic Instruments for Autism Spectrum Disorders in Toddlers. J. Autism Dev. Disord. 2006, 36, 839–847. [Google Scholar] [CrossRef]
- Constantino, J.N.; Davis, S.A.; Todd, R.D.; Schindler, M.K.; Gross, M.M.; Brophy, S.L.; Reich, W. Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. J. Autism Dev. Disord. 2003, 33, 427–433. [Google Scholar] [CrossRef]
- Dunn, W.; Daniels, D.B. Initial Development of the Infant/Toddler Sensory Profile. J. Early Interv. 2022, 25, 27–41. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.; Al-Drees, A.; Al-Arfaj, A. Effectiveness of Auditory Integration Therapy in Autism Spectrum Disorders--Prospective Study. Autism Insights 2013, 5, 13–20. [Google Scholar] [CrossRef]
- Perrin, J.M.; Coury, D.L.; Hyman, S.L.; Cole, L.; Reynolds, A.M.; Clemons, T. Complementary and Alternative Medicine Use in a Large Pediatric Autism Sample. Pediatrics 2012, 130 (Suppl. S2), S77–S82. [Google Scholar] [CrossRef]
- El-Ansary, A.; Hassan, W.M.; Daghestani, M.; Al-Ayadhi, L.; Ben Bacha, A. Preliminary evaluation of a novel nine-biomarker profile for the prediction of autism spectrum disorder. PLoS ONE 2020, 15, e0227626. [Google Scholar] [CrossRef] [PubMed]
- Heuer, L.S.; Croen, L.A.; Jones, K.L.; Yoshida, C.K.; Hansen, R.L.; Yolken, R.; Zerbo, O.; DeLorenze, G.; Kharrazi, M.; Ashwood, P.; et al. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol. Psychiatry 2019, 86, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Melnyk, S.; Macfabe, D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.; Zayed, N.; Bhat, R.S.; Moubayed, N.M.S.; Al-Muammar, M.N.; El-Ansary, A. The use of biomarkers associated with leaky gut as a diagnostic tool for early intervention in autism spectrum disorder: A systematic review. Gut Pathog. 2021, 13, 54. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef] [PubMed]
- Caia, J.; Ding, L.; Zhang, J.S.; Xue, J.; Wang, L.Z. Elevated plasma levels of glutamate in children with autism spectrum disorders. NeuroReport 2016, 27, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Tilleux, S.; Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007, 85, 2059–2070. [Google Scholar] [CrossRef]
- Domercq, M.; Perez-Samartin, A.; Aparicio, D.; Alberdi, E.; Pampliega, O.; Matute, C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 2009, 58, 730–740. [Google Scholar] [CrossRef]
- Pampliega, O.; Domercq, M.; Soria, F.N.; Villoslada, P.; Rodríguez-Antigüedad, A.; Matute, C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J. Neuroinflammation 2011, 8, 63. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Ben Bacha, A.G.; Al-Ayadhi, L.Y. Proinflammatory and proapoptotic markers in relation to mono and di-cations in plasma of autistic patients from Saudi Arabia. J. Neuroinflammation 2011, 8, 142. [Google Scholar] [CrossRef]
- Axtell, R.C.; Xu, L.; Barnum, S.R.; Raman, C. CD5-CK2 Binding/Activation-Deficient Mice Are Resistant to Experimental Autoimmune Encephalomyelitis: Protection Is Associated with Diminished Populations of IL-17-Expressing T Cells in the Central Nervous System. J. Immunol. 2006, 177, 8542–8549. [Google Scholar] [CrossRef] [PubMed]
- Sestero, C.M.; McGuire, D.J.; De Sarno, P.; Brantley, E.C.; Soldevila, G.; Axtell, R.C.; Raman, C. CD5-Dependent CK2 Activation Pathway Regulates Threshold for T Cell Anergy. J. Immunol. 2012, 189, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Mier-Aguilar, C.A.; Cashman, K.S.; Raman, C.; Soldevila, G. CD5-CK2 Signaling Modulates Erk Activation and Thymocyte Survival. PLoS ONE 2016, 11, e0168155. [Google Scholar] [CrossRef] [PubMed]
- Ulges, A.; Witsch, E.J.; Pramanik, G.; Klein, M.; Birkner, K.; Bühler, U.; Wasser, B.; Luessi, F.; Stergiou, N.; Dietzen, S.; et al. Protein kinase CK2 governs the molecular decision between encephalitogenic T H 17 cell and T reg cell development. Proc. Natl. Acad. Sci. USA 2016, 113, 10145–10150. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Antelo, M.; Serrano, M.P.; Manterola, A.; Ruiz, A.; Llavero, F.; Mato, S.; Zugaza, J.L.; Pérez-Cerdá, F.; Matute, C.; Sánchez-Gómez, M.V. Inhibition of Casein Kinase 2 Protects Oligodendrocytes from Excitotoxicity by Attenuating JNK/p53 Signaling Cascade. Front. Mol. Neurosci. 2018, 11, 333. [Google Scholar] [CrossRef]
- Hafizi, S.; Tabatabaei, D.; Lai, M.-C. Review of Clinical Studies Targeting Inflammatory Pathways for Individuals with Autism. Front. Psychiatry 2019, 10, 849. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Williams, Z.J.; Failla, M.D.; Gotham, K.O.; Woynaroski, T.G.; Cascio, C. Psychometric Evaluation of the Short Sensory Profile in Youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 4231–4249. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Boe, D.M.; Yu, D.; Weber, C.R.; Marchiando, A.M.; Bradford, E.M.; Wang, Y.; Wu, L.; Schneeberger, E.E.; Shen, L.; et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011, 193, 565–582. [Google Scholar] [CrossRef]
- White, A.; McGlone, A.; Gomez-Pastor, R. Protein Kinase CK2 and Its Potential Role as a Therapeutic Target in Huntington’s Disease. Biomedicines 2022, 10, 1979. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).