Abstract
A growing body of studies suggests that Ca2+ signaling controls a variety of biological processes in brain elements. Activation of L-type voltage-operated Ca2+ channels (VOCCs) plays a role in the development of oligodendrocyte (OL) lineage loss, and indicates that the blocking of these channels may be an effective way to inhibit OL lineage cell loss. For this study, 10.5-day-old male Sprague–Dawley rats were used to generate cerebellar tissue slices. The slice tissues were cultured and randomly allocated to one of four groups (six each) and treated as follows: Group I, (sham control); Group II, 0.1% dimethyl sulfoxide (DMSO) only (vehicle control); Group III, injury (INJ); Group IV, (INJ and treatment with NIF). The injury was simulated by exposing the slice tissues to 20 min of oxygen–glucose deprivation (OGD). At 3 days post-treatment, the survival, apoptosis, and proliferation of the OL lineages were measured and compared. Results: In the INJ group, there was a decrease in mature myelin basic protein+ OLs (MBP+ OLs) and their precursors, NG2+ OPCs (Nerve-glia antigen 2+ oligodendrocyte precursor cell), compared with controls. A significant elevation was observed in the NG2+ OPCs and apoptotic MBP+ OLs as confirmed by a TUNEL assay. However, the cell proliferation rate was decreased in NG2+ OPCs. NIF increased OL survival as measured by apoptosis rate in both OL lineages and preserved the rate of proliferation in the NG2+ OPCs. Conclusions: Activation of L-type VOCCs may contribute to OL pathology in association with reduced mitosis of OPCs following brain injury as a strategy to treat demyelinating diseases.
1. Introduction
Increasing evidence suggests that ischemic brain injury correlates with long-term neurological problems resulting in part from oligodendrocyte (OL) lineage damage and a loss of appropriate myelination [1]. CNS myelination disturbance is a central feature experienced in conditions ranging from ischemia, periventricular leukomalacia, and cerebral palsy in infants [2] to stroke and multiple sclerosis in adults [1,3]. These resulting conditions can leave affected individuals severely handicapped with little or no recovery prospects, yet no specific pharmacotherapies presently exist [4,5,6].
The various cellular brain elements are separately under ischemic attack [7]. The types of glia which are most vulnerable to injury are those of the OL lineages [8]. The precise underlying mechanisms that are involved in ischemic brain injury-induced OL loss are not fully understood. However, in recent years, studies suggest that OL lineages are vulnerable to glutamate excitotoxicity, reactive oxygen species (ROSs) with oxidative stress, release of nitric oxide, and elevated Ca2+ influx [9]. Brain ischemia can cause elevated glutamate levels in the extracellular space [10] largely due to the reversal of the glutamate transporters [7,11]. Glutamate is also released via cystine-glutamate anti-porter activity and vesicular release after depletion of ATP [12]. High amounts of glutamate and the resultant sustained activation of Ca2+-permeable ionotropic glutamate receptors are involved in subsequent OL death [2,13].
Ca2+ homeostasis is essential for the development and survival of many cell types including glial cells of the CNS [14]. The influx of Ca2+ through store-operated and voltage-operated Ca2+ channels (VOCCs) play an essential role in many cellular processes, such as cell migration and proliferation [15]. Immunocytochemical study showed that the expressions of L-, N-, and R-type VOCC channels in OL lineage cells stem from different brain regions [14,15]. Several processes are regulated by L-VOCCs, such as migration of OL precursor cells (OPCs) [16]. Ca2+ dyshomeostasis is related to CNS pathophysiology [14]. Evidence suggests that there is a relationship between excessive Ca2+ ion influx and cell death in various cell types [17]. Studies using optic nerve models, in which injury is induced by hypoxia–ischemia, showed that VOCCs participate in the cellular damage process [18]. In cells within the OL lineage, OPCs are extremely vulnerable to prolonged and excessive intracellular Ca2+ ion influx resulting from channel and pump dysregulation [16], or from Ca2+ that is released from internal stores, which alters the regulatory mechanisms of Ca2+. This leads to an inappropriate stimulation of Ca2⁺-dependent enzymes and processes and metabolic derangements, and triggers necrosis or apoptosis [15].
Damage to OL lineages results in a loss of appropriate myelination [19]. However, myelinating OL can repair myelin after injury-induced demyelination, and myelin repair derives from recruitment and differentiation of an endogenous population of OPCs [20,21,22,23,24]. The lineage of OLs traverses many distinct steps from OPC expressing a characteristic set of markers, including platelet-derived growth factor α receptor (PDGFαR) and the proteoglycan Nerve-glia antigen 2+ (NG2), to mature OLs expressing the myelin genes: myelin genes such as myelin-associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), 2′-3′-cyclic nucleotide 3′ phosphohydrolase (CNP), and proteolipid protein (PLP) and their product proteins [20,21,22]. In this study, we hypothesized that brain ischemia would activate L-type VOCCs and that treatment with nifedipine (NIF), a blocker of L-type Ca2+ channels, would minimize OL lineage pathology in an ex vivo model system of rat cerebellum. Our results indicate that blocking the L-type VOCC activity reduced MBP+ OL loss. This protection also correlated with the enhancement of survival and proliferation OL precursors (NG2+ OPCs). Our findings may provide insight into a pharmacological approach to prevent demyelinating diseases characterized by OL lineage loss.
2. Materials and Methods
2.1. Ethical Statement
Animal experiments were carried out in accordance with the regulations of the Research Ethics Committee University of Tripoli, Tripoli, Libya. Approval number (ref. BEC-BTRC 29-2020).
2.2. Animals and Housing Conditions
Male Sprague–Dawley rats (n = 24), aged 10.5 days old and weighing 17.1 ± 1.23 g, were obtained from the Faculty of Sciences at the University of Tripoli, Tripoli, Libya. Animals were maintained at an ambient temperature with a 12 h light/dark cycle. Animals were provided free access to a standard diet and water.
2.3. Preparation of Cerebellar Slice Tissues
Methods were based on previously published protocols [25,26,27]. In brief, male, 10.5-day-old Sprague–Dawley rats (n = 24) weighing 17.1 ± 1.23 g were used to generate cerebellar slice tissues. The animals were sacrificed, and the cerebellum was dissected and transversely sliced at a thickness of 300 µm on a vibratome (Leica, Germany). Eight to ten tissue slices per rat brain (6 animals per each experimental group) were carefully transferred onto humidified 1.0 µm pore size cell culture inserts (Millipore, Falcon, Bristol, UK) and placed in a 6-well plate (Falcon, Bristol, UK). The slice tissues were kept in one ml of serum-based medium (50% minimum essential medium Eagle (MEME, Sigma, Bristol, UK), 25% HBSS (hanks balanced salt solution, Invitrogen, Bristol, UK), 20% normal horse serum (Invitrogen), 4.6 mM, (v/v) L-glutamine (Sigma), 21 mM (v/v) D-glucose (Fisher Scientific, Bristol, UK), 1% penicillin/streptomycin solution (Invitrogen), 4.2 µM (v/v) L-ascorbic acid (Aldrich-Sigma, Schnelldorf, Germany), and 11 mM (v/v) NaHCO3 at pH 7.2–7.4) in a humidified aerobic incubator (5% CO2) at 37 °C for 3 days. The culture medium was replaced with serum-free medium supplemented with 0.3% B27 growth supplement (Invitrogen).
2.4. Induction of Cerebellar Slice Tissue Injury
Cerebellar slice tissue injury was simulated by exposing 3-day-old slice tissue cultures to 20 min oxygen–glucose deprivation (OGD) insult. Briefly, the cultured tissues were transferred into filter-sterilized, deoxygenated glucose-free culture medium for 20 min in an anaerobic airtight chamber with a mixture of 95% N2/5% CO2 gas flow, temperature maintained at 37 ± 0.5 °C. After OGD insult, the cultures were washed at least three times with fresh oxygenated culture medium containing 5 mg/mL D-glucose and supplemented with 0.3% B27 and returned to their culture conditions under normoxic atmosphere (5% CO2) at 37 °C. Non-OGD treated tissue cultures (sham and vehicle control cultures) were maintained for the same time under normoxic atmosphere (5% CO2) at 37 °C. To mimic in vivo conditions, the cultures were incubated for 3 days before being fixed for analysis.
2.5. Experimental Design
The tissue culture slices were randomly allocated to one of 4 groups (6 each) and treated as follows: Group I (sham control), the cultured tissue slices were cultured under normoxic conditions at 37 ± 0.5 °C at the time points corresponding to those in other experimental groups; Group II (vehicle control), the cultured tissue slices were treated with vehicle alone (0.1% DMSO); Group III (injury; INJ), the cultured tissue slices were cultured and subjected to 20 min of OGD insult in an atmospheric perfusion airtight chamber (95% N2/5% CO2) gas flow at 37 ± 0.5 °C. After OGD treatment, the cultures were washed three times with normal medium before returning them to normoxic atmosphere for 3 days of reperfusion; Group IV, (INJ and treatment with blocker NIF), cultured tissue slices were subjected to 20 min of OGD insult and then were treated with the blocker NIF (10 μM). The blocker was added to culture medium 20 min after the OGD end and maintained in the culture medium for 60 min. Appropriate control groups were performed at the corresponding time points with this blocker to test their cytotoxicity.
2.6. Cell Viability Assay
Cell viability was assessed using a commercial kit (Molecular Probes, Invitrogen, Darmstadt, Germany) according to the manufacturer’s instructions. Briefly, cultured brain tissues were incubated in the presence of a solution containing 2 µM calcein-AM (Cal, green) and 4 µM ethidium homodimer-AM (EthD-1, red) at 37 °C for 20 min and fixed in paraformaldehyde (4%) (Sigma, Germany) in PBS (Oxoid, Germany) for 10 min at room temperature.
2.7. TUNEL Assay
DNA fragmentation was assessed using a commercial kit (Chemicon ApopTag Fluorescein in Situ Apoptosis Detection kit, S7110 Sigma-Aldrich, UK) according to the manufacturer’s instructions. Tissue sections were incubated with TDT enzyme for one hour at 37 °C in the dark and the reaction was terminated with a stop/wash buffer. Following washing, sections were stained with DAPI (4,6-Diamidino-2-Phenylindole) (Vector Laboratories, Bristol, UK) to label nuclei. To identify the identity of the TUNEL-positive cells, co-localization between TUNEL staining and MBP or NG2 immunostaining was determined.
2.8. Immunocytochemistry
For immunocytochemical analysis, cerebellar slice cultures were fixed in 4% PFA in PBS for 60 min at room temperature. Following three washes with PBS, the slices were mounted onto glass slides and blocked for 1 h in a serum-blocking solution containing 10% normal goat serum (MP Biomedical, Bristol, UK) and 0.25% Triton 100-X (Sigma, UK) in PBS. Primary antibodies were diluted in PBS and incubated for 24 h at 4 °C in the dark. The slides were washed three times for 5 min each with PBS and incubated with secondary antibodies diluted in PBS for 2 h at room temperature in the dark, followed by three washes in PBS. The primary antibodies used were MBP (myelin basic protein) (1:200 in PBS; MBL), NG2 (Nerve-glia antigen 2+) (1:300 in PBS; Millipore), and BrdU (1:1000 in PBS; Abcam, Bristol, UK). The secondary antibodies used for immunocytochemistry were Alexa Fluor® 633 and 488 (1:100; Invitrogen, Darmstadt, Germany).
2.9. Cell Proliferation Assay
Cell proliferation was measured by BrdU (5-bromo-2′-deoxyuridine, Aldrich-Sigma, Gillingham, UK) assay [28]. Cultured tissue slices were incubated in 20 µM BrdU-containing medium for 3 or 24 h prior to fixation. The fixed cultures were incubated with 1N HCl for 10 min on ice followed by a 10-min incubation with 2N HCl at room temperature and placed in an incubator at 37 °C for 20 min. Acidic conditions were neutralized by placing the slides in borate buffer (0.1 M, pH = 8.5) for 12 min. Slides were washed three times for 5 min each with PBS and 1% Triton 100-X and permeabilized in a solution containing PBS (1 M) with 1% Triton 100-X, glycine (1 M), and normal goat serum (5%) for 1 h. The slides were then incubated overnight with anti-BrdU mono-antibody (eBioscience, Oxford, UK) at a dilution of 1: 50 in PBS followed by DAPI staining of the nuclei.
2.10. Cell Quantification
Using confocal microscopy, tissue sections were scanned, imaged, and analyzed. Cell scoring was performed as previously described by Al-Griw et al. (2021). In brief, cell counts were performed across a minimum of nine predetermined grid sections outlined by hand using imageJ (Grid size, 400 × 300 µm; Counting frame, 25 × 25 µm; z-depth 50 µm) at 40× or 63× magnifications in 8 to 10 randomly selected fields per slice with three slices per animal per condition.
2.11. Statistics
The data were analyzed using GraphPad Prism software (version 7.0). All data were presented as the mean ± SEM for at least five independent experiments, each performed in triplicate. Normality was determined by the computerized Kolmogorov–Smirnov test. Intergroup comparison of normally distributed data was performed with a Student’s paired t-test and multiple comparisons were made using a one-way ANOVA followed by a post hoc test for multiple comparisons and Dunnett’s multiple-comparisons tests to detect pairwise intergroup differences. p < 0.05 was considered statistically significant.
3. Results
3.1. L-Type Ca2+ Channel Inhibitor NIF Preserves Cell Viability following Brain Tissue Injury
Here, we identified the effect of L-type VOCC inhibition on cell viability after brain tissue injury. The results indicated the presence of viable cells (Cal, green, white arrow heads) and dead cells (EthD-1, red, asterisks) in all experimental groups (Figure 1A). Specifically, we found that cell viability was significantly lower (p < 0.001; Figure 1B) in the INJ group (25.3 ± 3.49%) compared with the sham group (78.95 ± 1.75% per cubic micrometer), and the L-type Ca2+ inhibitor, NIF (10 μM), preserved cell viability (72.47 ± 2.63%, p < 0.0001; Figure 1B), whereas treatment with vehicle alone showed no effect when applied under controlled conditions.
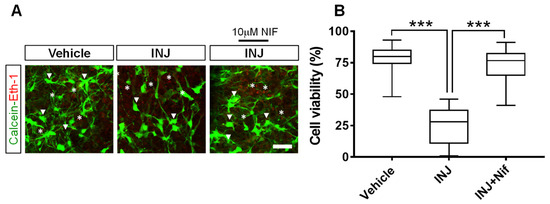
Figure 1.
NIF enhances neural cell viability following injury. Tissue sections representing controls, INJ alone, or treatment with NIF. (A) Immunofluorescent images of viable cells (calcein-AM (Cal) green, white arrow heads) and nuclei of dead cells (ethidium homodimer-AM (EthD-1) red, asterisks). (B) Quantification of cell viability per cubic micrometer. Data are presented as the mean ± SEM of 8 to 10 slices/animal with 6 animals per experimental group. (***) indicates p < 0.001.
3.2. L-Type Ca2+ Channel Inhibitor NIF Minimizes Apoptosis following Brain Tissue Injury
We determined whether NIF treatment-induced protection following brain tissue injury was associated with alterations in apoptosis (pyknotic nuclei). A TUNEL assay in combination with DAPI-nuclei labeling was carried out. The results indicated that in the INJ group, there was increased apoptosis compared with that in the sham group (Figure 2A). Specifically, we found that apoptotic (TUNEL+) cells were increased in number in the INJ group compared with the sham group (38.67 ± 2.17% vs. 9.34 ± 1.04%, p < 0.0001; Figure 2B). Treatment with 10 μM NIF markedly reduced apoptosis (12.09 ± 1.29%, p < 0.001; Figure 2B).
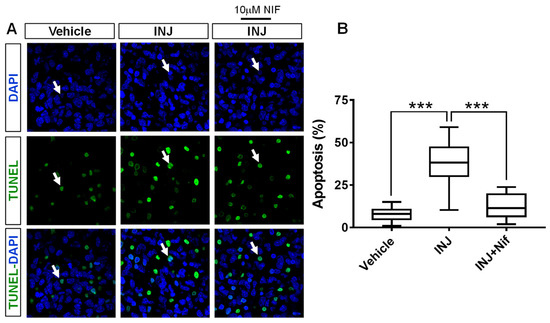
Figure 2.
NIF reduces apoptosis following brain tissue injury. Tissue sections representing controls, INJ alone, or treatment with NIF. (A) Immunofluorescent images showing apoptotic cells (green, white arrow heads). Scale bar: 20 µm. (B) Assessment of apoptosis. Data are presented as the mean ± SEM of 8 to 10 slices/animal with 6 animals per experimental group. (***) indicates p < 0.001.
3.3. L-Type Ca2+ Channel Inhibitor NIF Restores Mature OL Survival following Brain Tissue Injury
Because Ca2+ ion influx through L-type VOCCs mediates cell loss in many model systems [29,30], we postulated that blocking L-type VOCC activity may protect maturing OLs. The MBP primary antibody was used for immunocytochemical analysis. No significant shift in morphology and density of MBP+ OL nuclei was observed in the absence of injury (Figure 3A, white arrow heads). Uninjured MBP+ OLs were dimly fluorescent with oval cell bodies containing three–five nuclear inclusions in a clear cytoplasm. In contrast, the INJ group exhibited a significant MBP+ OL loss (Figure 3A,B). The extent of insult rapidly reduced the number of MBP+ OLs to 33.24 ± 8.26/mm2 compared with 134.8 ± 14.21/mm2 for control conditions (p = 0.0003; Figure 3B). In contrast, treatment with 10 μM NIF effectively preserved the number of MBP+ OLs (90.89 ± 5.77/mm2, p = 0.0403; Figure 3B).
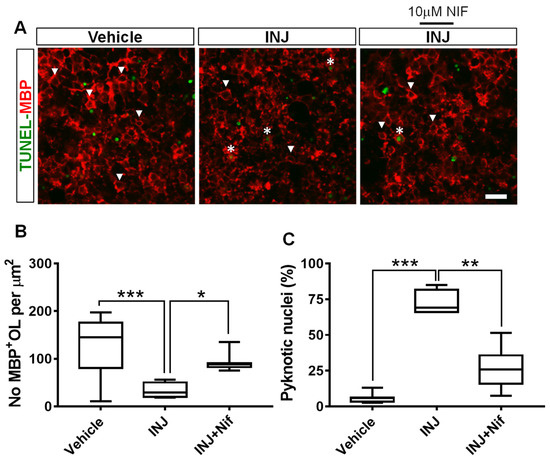
Figure 3.
NIF reduces MBP+ OL loss following brain tissue injury. Tissues representing controls, INJ alone, or treatment with NIF. (A) Immunofluorescent images of MBP+ OLs (white arrowheads) and condensed and fragmented nuclei (white asterisks). Scale bar: 20 µm. (B) Measurement of MBP+ OLs. (C) Quantification of apoptotic nuclei among MBP+ OLs. Data shown are the mean ± SEM of 8 to 10 slices/animal with 6 animals per group. * indicates p < 0.05; ** indicates p < 0.01, and *** indicates p < 0.001.
To determine whether NIF treatment could decrease apoptosis in MBP+ OLs in this model system, a TUNEL assay in combination with MBP immunostaining was carried out. Compared with the controls, the INJ group exhibited a significant augmentation in the pyknotic nuclei in the MBP+ OLs (Figure 3A, asterisks). Moreover, pyknotic nuclei in the MBP+ OLs increased from 6.42 ± 2.67% to 77.95 ± 5.35% (p < 0.001; Figure 3B). NIF treatment markedly reduced the density of apoptotic MBP+ OLs (36.01 ± 7.35%, p = 0.0022; Figure 3C).
3.4. L-Type Ca2+ Channel Inhibitor NIF Enhances NG2+ OPC Survival following Injury
OPCs are capable of repairing demyelinated tissue since they can self-renew and mature into myelin-producing OLs cells of the CNS [31]. To determine whether the blocking of L-type VOCC activity affects OPC density and nuclear morphology, we performed a TUNEL assay in combination with immunocytochemical analysis for the early OPC stage-specific marker, NG2. Under control conditions, there was no marked variation in NG2+ OPC nuclear morphology or density (Figure 4A, white arrow heads). In the INJ group, only a few individual NG2+ OPCs with dim staining were observed (Figure 4A, white arrow heads). The morphology of OPCs conformed to prior descriptions and were characterized by small, irregular, rounded, cell bodies with a few short processes that were highly branched [32]. Furthermore, the INJ group exhibited a significant NG2+ OPC loss and a gain in pyknotic nuclei exhibiting brighter TUNEL fluorescence (Figure 4A). The period of insult markedly (p = 0.0002) reduced the counts of NG2+ OPCs to 47.5 ± 3.64 (Figure 4B) compared with 111.1 ± 9.86 for control conditions. In contrast, treatment with 10 μM NIF effectively preserved the count of NG2+ OPCs (83.16 ± 4.83/mm2, p = 0.03; Figure 4B). Using TUNEL assay, it was shown that pyknotic nuclei among NG2+ OPC population increased from 5.63 ± 0.82% to 66.72 ± 4.44% (p = 0.012; Figure 4B, asterisks). Ten μM NIF treatment effectively preserved NG2+ OPC numbers and restricted numbers of pyknotic nuclei compared to INJ group (48.16 ± 3.47%; Figure 4C).
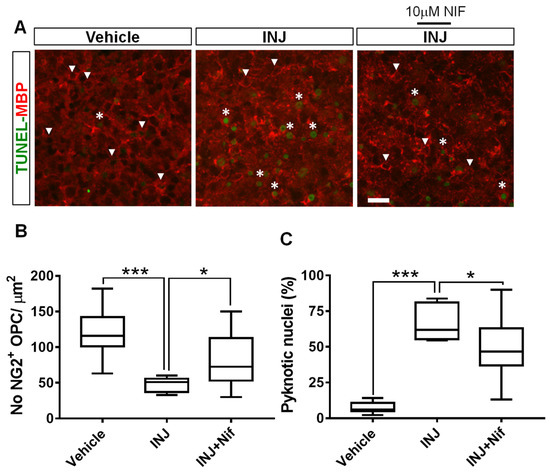
Figure 4.
NIF reduces NG2+ OPC loss following insult. Tissue sections representing controls, INJ alone, or treatment with NIF. (A) Immunofluorescent images of NG2+ OPCs (white arrowheads) and pyknotic nuclei (white asterisks). Scale bar: 20 µm. (B) Measurement of NG2+ OPCs. (C) Quantification of apoptosis. Data are shown as the mean ± SEM of 8 to 10 slices/animal with 6 animals per each experimental group. * indicates p < 0.05 and *** indicates p < 0.001.
3.5. L-Type Ca2+ Channel Inhibitor NIF Preserves NG2+ OPC Mitotic Behavior following Injury
The effect of NIF on cell mitosis in response to injury was examined. The DNA replication marker, BrdU, was used. The assay showed a significant (p < 0.0001; Figure 5B, asterisks) decrease in the INJ group of dividing (BrdU+) cells compared with the sham group. The 10 μM NIF treatment markedly preserved cell mitotic behavior compared with the INJ group (p = 0.022, Figure 5B).
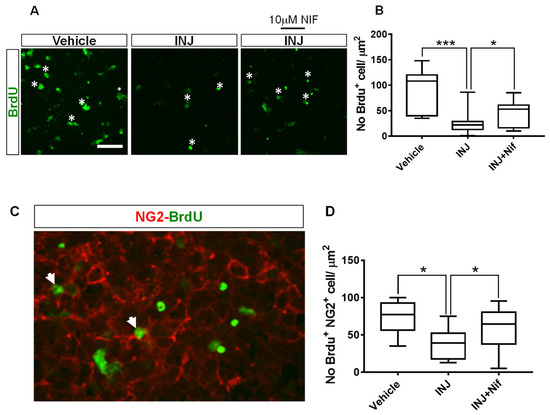
Figure 5.
NIF preserves mitotic behavior of NG2+ OPCs following insult. Tissue sections representing controls, INJ alone, or treatment with NIF. Immunofluorescence images of (A) dividing (BrdU+) cells (white asterisks) and (C) proliferating NG2+ OPCs (white arrowheads). Scale bar: 20 µm. (B) Measurement of BrdU+ cells. (D) Measurement of proliferating NG2+ OPCs. Data are presented as the mean ± SEM of 8 to 10 slices/animal with 6 animals per each experimental group. * indicates p < 0.05; *** indicates p < 0.001.
Because the inhibition of L-type VOCCs with NIF improved OPC survival in this model, we presumed that 10 μM NIF treatment could affect the proliferation of OPCs. To test this, a BrdU assay in combination with immunostaining of an early OPC marker, NG2, was performed. We found that compared with the control, proliferating NG2+ OPCs were less abundant in the INJ group and NIF treatment markedly enhanced their density (Figure 5C, white arrow heads). Specifically, in the INJ group, there was a significant (p = 0.018) decline in the percentage of BrdU+/NG2+ cells to 36.64 ± 4.91% compared with 68.05 ± 9.41% for the control group (Figure 5C). The 10 μM NIF treatment significantly protected the mitotic behavior of NG2+ OPCs (59.66 ± 7.38%, p = 0.036; Figure 5C).
4. Discussion
The findings of this study showed that blockade of L-type VOCC activity alleviated OL lineage pathology after ischemic brain tissue. Specifically, the L-type Ca2+ channel inhibitor, NIF, reduced apoptosis in MBP+ OLs. In addition, NIF enhanced the mitotic behavior and survival of NG2+ OPCs in an ex vivo model system of rat cerebellum. To our knowledge, this is the first demonstration that blocking L-type VOCCs after brain tissue injury protects OL lineages. Hence, the present study highlights the participation of L-type VOCC signaling in the brain in brain injury as the importance of VOCCs in signaling is well known, and suggests a strategy to treat demyelinating diseases.
Cell-based systems have been widely used to investigate the efficacy of a range of agents to treat brain tissue injury [25,33]. Our ex vivo model system of rat cerebellum provides a more suitable CNS environment with a mixed population of different cell types as opposed to studies of homogeneous cell cultures [25,27,28]. Neurodevelopmental deficits constitute a complex interplay of neurons and glia; thus, employing intact brain tissues retains a heterogeneous cell population and the interactions in a more complex multicellular environment may be studied [26]. In the present study, brain tissue injury was induced by subjecting tissue to a brief OGD insult followed by 72 h of reperfusion to mimic the in vivo event [34]. Reperfusion after transient OGD insult results in neural cell loss caused by disruption of cell membrane permeability [34].
The various cellular brain elements are separately under ischemic attack [7]. The precise underlying mechanisms that are involved in ischemic brain injury-induced neural cell loss are not fully understood. However, in recent years, studies suggest that OL lineages are vulnerable to glutamate excitotoxicity, reactive oxygen species (ROSs) with oxidative stress, release of nitric oxide, and elevated Ca2+ influx [9]. Brain ischemia can cause elevated glutamate levels in the extracellular space [10,27], largely due to the reversal of the glutamate transporters [7,11,27]. High amounts of glutamate and the resultant sustained activation of Ca2+-permeable ionotropic glutamate receptors is involved in subsequent OL death [2,13].
Signaling mechanisms of Ca2+ influx and efflux in addition to Ca2+ binding proteins play diverse roles in the expression of dependent genes, cell survival, proliferation, and differentiation [35]. Transporters of Ca2+, VOCCs, ligand gated Ca2+ channels, Ca2+ binding proteins, and store-operated Ca2+ channels maintain normal Ca2+ concentrations and gradients, which are important for normal Ca2+ signaling [36]. VOCCs regulate the entry of Ca2+ into and from a cell, responding to depolarization of the plasma membrane and converting an electrical signal into a chemical one [36]. VOCCs are multi-subunit structures which include a pore-forming subunit [3]. The pore-forming subunit is encoded by five types of VOCCs and depends on approximately 10 genes [36,37]. VOCC types include high voltage-activated channels (R- N-, L-, and P/Q types), whereas the low voltage-activated channel consists of the T-type.
Increased Ca2+ influx through VOCCs plays an essential role in triggering either necrosis or apoptosis [16], which can subsequently activate downstream signaling pathways to exert pro-survival effects [34,38]. A number of preventive modalities have been used to protect cells and tissues after injury or stress and followed by reperfusion, including maintenance of intracellular Ca2+ levels [34]. Here, we did not ascertain which specific neural cell type was predominantly affected, the extent of cell loss from neurons or glia, or changes to neuronal and glial interactions. Nonetheless, we found that the L-type VOCC inhibitor, NIF, enhanced neural viability and reduced apoptosis among neural cells. This suggests that blocking L-type VOCC activity may preserve all cell types prone to injury-induced cell death. Our findings illustrate that the L-type VOCC inhibitor, NIF, inhibits apoptosis in the neural cell population in our model system. Therefore, we propose that consistent protection through L-type VOCC inhibition following injury may be attributed to a drug-mediated increase in pro-survival and anti-apoptotic gene expression during the progression of brain tissue injury; however, it may also target excitotoxic and oxidative pathways.
Ischemic brain injury occurs at several distinct sites including axon and glia. Glia are endowed with Ca2+-permeable store-operated channels which can be activated through VOCCs, which are activated in response to depolarization of cell membranes, for example, because of an increase in extracellular K+ levels [39], all of which determine Ca2+ homeostasis [9,14]. As mentioned above, many studies indicate that there is a relationship between excessive Ca2+ influx and cell death [40]. Ca2+ homeostasis regulation plays an essential role in OL physiology, determining proliferation, migration, differentiation and myelination, damage, repair, or death [14,16,41]. For the normal development of OPCs, modulation of intracellular Ca2+ levels by L-type Ca2+ channels is important [16]. The types of glia which are most vulnerable to injury are those of the OL lineages [8]. OL lineages appear to be highly vulnerable to prolonged and excessive intracellular Ca2+ influx resulting from channel and pump dysregulation [42] or even Ca2+ release from internal stores which alters Ca2+ regulatory mechanisms. In cells within the OL lineage, OPCs are shown to be extremely vulnerable to prolonged and excessive intracellular Ca2+ ion influx resulting from channel and pump dysregulation, or from Ca2+ that is released from internal stores, which alters the regulatory mechanisms of Ca2+ [16]. Studies demonstrated the presence of N-, L-, and R-type channels in OLs [16,43]. Furthermore, studies in optic nerve models, in which injury is induced by hypoxia-ischemia, have revealed that VOCCs participate in cellular damage [44,45]. Therefore, we hypothesized that simulated brain tissue injury by OGD insult damages OLs lineages by activating L-type VOCCs and that the L-type Ca2+ channel inhibitor, NIF, can mitigate OL pathology shortly (20 min) following the insult. Our findings showed that blockade of the L-type Ca2+ channel activity after ischemic insult is associated with the preservation of mature OLs (MBP+ cells) in our model system. In addition, the L-type Ca2+ channel inhibitor, NIF, minimized apoptosis of the OL lineages. Additionally, we found that the attenuation of L-type VOCC activity by NIF enhanced cell viability and minimized apoptosis in immature OL populations (NG2+ OPCs). In addition, NIF preserved the mitotic behavior of NG2+ OPCs following injury. Taken together, these findings suggest that inhibition of L-type VOCC activity may impart robust protection and can be effective for the treatment of various neurodegenerative diseases [16]. This suggests that L-type VOCC-mediated Ca2+ influx is crucial to OL pathology.
5. Conclusions
This work highlights the therapeutic value of blocking L-type VOCC activity, specifically, in preventing or/and reversing OL pathology associated with a number of demyelinating diseases. It is clear from published clinical trials that no effective therapeutic strategy is available in the setting of neonatal brain tissue injury. The occurrence of ischemic brain injury is usually unpredictable and often renders pre-treatment impossible. The efficacy of the selective L-type Ca2+ channel blocker NIF as a delaying treatment strategy to treat demyelinating diseases, however, remains unclear. Our findings demonstrate that post-treatment with NIF at a time point immediately after injury attenuated OL loss. This suggests the persistence of L-type VOCC activity after the end of the insult. Because premature infants are generally maintained in a constantly monitored neonatal intensive care unit, blockade activity of L-type VOCCs even within a few minutes post-insult is also feasible to at least minimize brain damage.
Author Contributions
Conceptualization, M.A.A.-G. and R.A.; methodology, M.A.A.-G., R.A., H.W.R., M.E.B.-O. and R.T.; software, M.A.A.-G. and R.A.; validation, M.A.A.-G., R.A., W.S.A. and G.S.; formal analysis, G.S., M.M.H., A.A.E., A.M.A., N.A.B., A.A.-F. and W.S.A.; investigation, M.A.A.-G., H.W.R., R.A., R.T. and M.E.B.-O.; resources, R.A., R.T. and M.E.B.-O.; data curation, H.W.R., M.E.B.-O., A.A.E., A.M.A., N.A.B., A.A.-F. and W.S.A.; writing—original draft preparation, M.A.A.-G., R.A., H.W.R., M.E.B.-O. and R.T.; writing—review and editing, G.S., M.M.H., A.A.E., A.M.A., N.A.B., A.A.-F. and W.S.A.; visualization, M.A.A.-G., R.A., H.W.R., M.E.B.-O. and R.T.; supervision, G.S., M.M.H., A.A.E., A.M.A., N.A.B., A.A.-F. and W.S.A.; project administration, M.A.A.-G. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the University of Tripoli (ref. BEC-BTRC 29-2020).
Data Availability Statement
Data generated or analyzed during the current study are available from the corresponding author and included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001, 50, 553–562. [Google Scholar] [CrossRef]
- Clarke, G.; Aatsinki, A.; O’Mahony, S.M. Brain development in premature infants: A bug in the programming system? Cell Host Microbe 2021, 29, 1477–1479. [Google Scholar] [CrossRef]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef]
- Sherwin, C.; Fern, R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: Role of TNF-A, IL-1B, and calcium. J. Immunol. 2005, 175, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Han, B.H.; Luo, N.L.; Chricton, C.A.; Xanthoudakis, S.; Tam, J.; Arvin, K.L.; Holtzman, D.M. Selective vulnerability of late oligodendrocyte progenitors to hypoxia–ischemia. J. Neurosci. 2002, 22, 455–463. [Google Scholar] [CrossRef]
- Tekkök, S.B.; Ye, Z.; Ransom, B.R. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J. Cereb. Blood Flow Metab. 2007, 27, 1540–1552. [Google Scholar] [CrossRef]
- Matute, C.; Alberdi, E.; Domercq, M.; Sánchez-Gómez, M.V.; Pérez-Samartín, A.; Rodríguez-Antigüedad, A.; Pérez-Cerdá, F. Excitotoxic damage to white matter. J. Anat. 2007, 210, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Matute, C. Glutamate and ATP signalling in white matter pathology. J. Anat. 2011, 219, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.E. Role of glutamate receptors in periventricular leukomalacia. J. Child Neurol. 2005, 20, 950–959. [Google Scholar] [CrossRef]
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 2000, 403, 316–321. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, T.M.; Kabakov, A.Y.; Goldhoff, P.E.; Volpe, J.J.; Rosenberg, P.A. Regulation of glutamate transport in developing rat oligodendrocytes. J. Neurosci. 2009, 29, 7898–7908. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.G.; Fern, R. The mechanisms of acute ischemic injury in the cell processes of developing white matter astrocytes. J. Cereb. Blood Flow Metab. 2007, 28, 588–601. [Google Scholar] [CrossRef]
- Matute, C. Calcium dyshomeostasis in white matter pathology. Cell Calcium 2010, 47, 150–157. [Google Scholar] [CrossRef]
- Stys, P.K. White matter injury mechanisms. Curr. Mol. Med. 2004, 18, 113–130. [Google Scholar] [CrossRef]
- Paez, P.M.; Fulton, D.; Colwell, C.S.; Campagnoni, A.T. Voltage-operated Ca2+ and Na+ channels in the oligodendrocyte lineage. J. Neurosci. Res. 2009, 87, 3259–3266. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Tuo, Q.-Z.; Zhang, S.-T.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2022, 42, 259–305. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp. Transl. Stroke Med. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Reynoldsand, R.; Fawcett, J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001, 24, 39–47. [Google Scholar] [CrossRef]
- McTigue, D.M.; Wei, P.; Stokes, B.T. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 2001, 21, 3392–3400. [Google Scholar] [CrossRef]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–926. [Google Scholar] [CrossRef]
- Suyama, K.; Watanabe, M.; Sakai, D.; Osada, T.; Imai, M.; Mochida, J. Nkx2.2 expression in differentiation of oligodendrocyte precursor cells and inhibitory factors for differentiation of oligodendrocytes after traumatic spinal cord injury. J. Neurotrauma 2007, 24, 1013–1025. [Google Scholar] [CrossRef]
- Al-Griw, M.A.; Alghazeer, R.O.; Awayn, A.; Shamlan, G.; Eskandrani, A.A.; Alnajeebi, A.M.; Babteen, N.A.; Alansari, W.S. Selective adenosine A2A receptor inhibitor SCH58261 reduces oligodendrocyte loss upon brain injury in young rats. Saudi J. Biol. Sci. 2021, 28, 310–316. [Google Scholar] [CrossRef]
- Al-Griw, M.A.; Wood, I.C.; Salter, M.G. Cerebellar Organotypic Slice Culture System: A Model of Developing Brain Ischaemia. Life Sci. J. 2017, 14, 89–98. [Google Scholar]
- Al-Griw, M.A.; Salter, M.G.; Wood, I.C. Inhibition of ionotropic GluR signaling preserves oligodendrocyte lineage and myelination in an ex vivo rat model of white matter ischemic injury. Acta Neurobiol. Exp. 2021, 81, 233–248. [Google Scholar] [CrossRef]
- Al-Griw, M.A.; Salter, M.G.; Wood, I.C. Blocking of NF-kB/p38MAPK pathways mitigates oligodendrocyte pathology in a model of neonatal white matter injury. Acta Neurobiol. Exp. 2022, 82, 52–64. [Google Scholar]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Meco, E.; Zheng, W.S.; Sharma, A.H.; Lampe, K.J. Guiding oligodendrocyte precursor cell maturation with Urokinase plasminogen activator-degradable elastin-like protein hydrogels. Biomacromolecules 2020, 21, 4724–4736. [Google Scholar] [CrossRef]
- Levine, J.M.; Card, J. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: Association with smooth protoplasmic astrocytes. J. Neurosci. 1987, 7, 2711–2720. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Histone deacetylase in neuropathology. Adv. Clin. Chem. 2021, 104, 151–231. [Google Scholar]
- Shi, Z.; Wu, D.; Yao, J.P. Protection against Oxygen-Glucose Deprivation/Reperfusion Injury in Cortical Neurons by Combining Omega-3 Polyunsaturated Acid with Lyciumbarbarum Polysaccharide. Nutrients 2016, 8, 41. [Google Scholar] [CrossRef]
- Pietrobon, D. Calcium channels and channelopathies of the central nervous system. Mol. Neurobiol. 2002, 25, 31–50. [Google Scholar] [CrossRef]
- Boczek, T.; Zylinska, L. Receptor-Dependent and Independent Regulation of Voltage-Gated Ca2+ Channels and Ca2+-Permeable Channels by Endocannabinoids in the Brain. Int. J. Mol. Sci. 2021, 22, 8168. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Ureshino, R.P.; Rocha, K.K.; Lopes, G.S.; Bincoletto, C.; Smaili, S.S. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid. Redox Signal 2014, 21, 123–137. [Google Scholar] [CrossRef]
- Alberdi, E.; Sanchez-Gomez, M.V.; Matute, C. Calcium and glial cell death. Cell Calcium 2005, 38, 417–425. [Google Scholar] [CrossRef]
- Cho, S.G.; Choi, E.J. Apoptotic signaling pathways: Caspases and stress-activated protein kinases. J. Biochem. Mol. Biol. 2002, 35, 24–27. [Google Scholar] [CrossRef]
- Butt, A.M.; Bay, V. Axon-glial interactions in the central nervous system. J. Anat. 2011, 219, 1. [Google Scholar] [CrossRef]
- Cheli, V.T.; Santiago González, D.A.; Spreuer, V.; Paez, P.M. Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cells maturation and myelination in vitro. Exp. Neurol. 2015, 265, 69–83. [Google Scholar] [CrossRef]
- Butt, A.M. Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia 2006, 54, 666–675. [Google Scholar] [CrossRef]
- Fern, R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J. Neurosci. 1998, 18, 7232–7243. [Google Scholar] [CrossRef]
- Imaizumi, T.; Kocsis, J.D.; Waxman, S.G. The role of voltage-gated Ca21 channels in anoxic injury of spinal cord white matter. Brain Res. 1999, 817, 84–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).