Perspective for Studying the Relationship of miRNAs with Transposable Elements
Abstract
1. Introduction
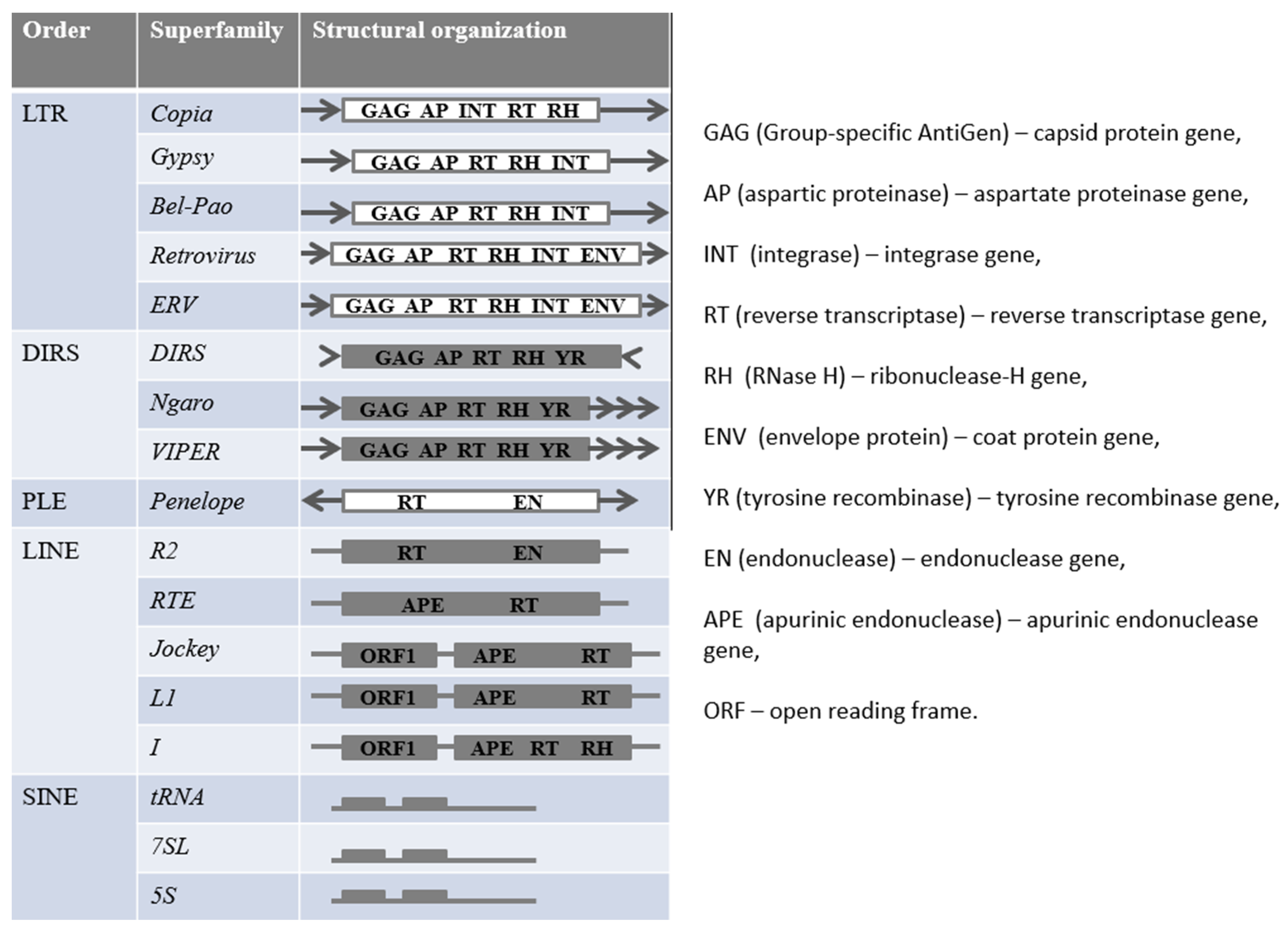
2. Differences of the Origin of miRNA from Transposons in Plants from Animals
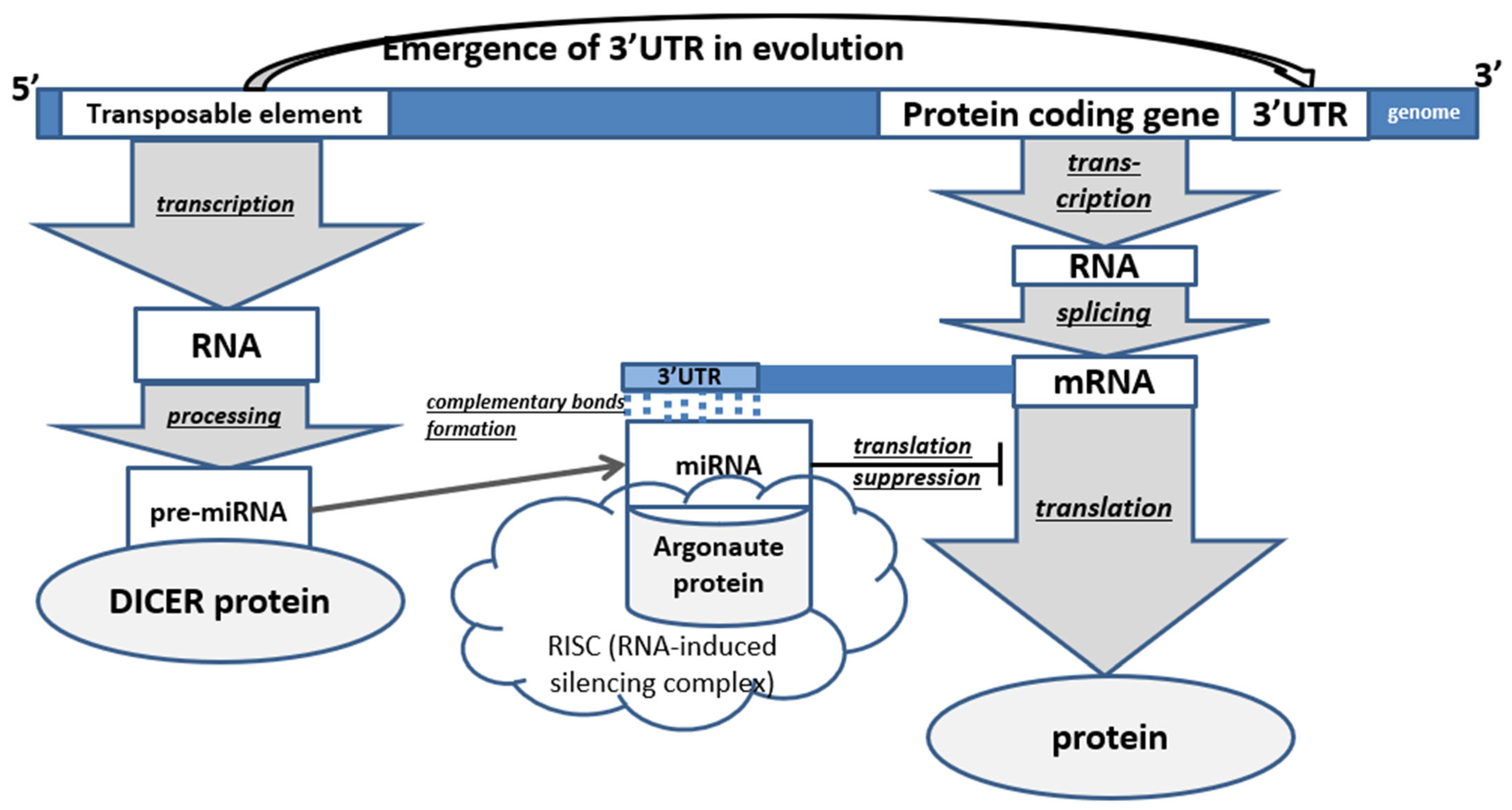
3. The Role of Transposons in the Emergence of miRNA in Animals
4. Prospects for the Creation of a Human Transposable Elements-Derived miRNA Database
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannan, S.; Chernikova, D.; Rogozin, I.B.; Poliakov, E.; Managadze, D.; Koonin, E.V.; Milanesi, L. Transposable Element Insertions in Long Intergenic Non-Coding RNA. Front. Bioeng. Biotechnol. 2015, 37, 71. [Google Scholar] [CrossRef]
- Shabalina, S.A.; Koonin, E.V. Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. 2008, 23, 578–587. [Google Scholar] [CrossRef]
- Cho, J. Transposon-Derived Non-coding RNAs and Their Function in Plants. Front. Plant Sci. 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Feschotte, C. The contribution of transposable elements to the evolution of regulatory networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Seal, R.L.; Chen, L.L.; Griffiths-Jones, S.; Lowe, T.M.; Mathews, M.B.; O’Reilly, D.; Prierce, A.J.; Stadler, P.F.; Ulitsky, I.; Wolin, S.L.; et al. A guide to naming human non-coding RNA genes. EMBO J. 2020, 39, e103777. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tian, J.; Mo, B. siRNA-mediated DNA methylation and H3K9 dimethylation in plants. Protein Cell 2013, 4, 656–663. [Google Scholar] [CrossRef]
- Chalertpet, K.; Pin-On, P.; Aporntewan, C.; Patchsung, M.; Ingrungruanglert, P.; Israsena, N.; Mutirangura, A. Argonaute 4 as an Effector Protein in RNA-Directed DNA Methylation in Human Cells. Front. Genet. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, U.; Srivastava, S.; Khatri, I.; Nanda, J.S.; Subramanian, S.; Arora, A.; Singh, J. Ablation of RNA interference and retrotransposons accompany acquisistion and evolution of transposases to heterochromatin protein CENPB. Mol. Biol. Cell 2017, 28, 1132–1146. [Google Scholar] [CrossRef]
- Samantarrai, D.; Dash, S.; Chhetri, B.; Mallick, B. Genomic and epigenomic cross-talks in the regulatory landscape of miRNAs in breast cancer. Mol. Cancer Res. 2013, 11, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.J.; Yi, X.; Zhao, X.W.; Zhao, Y.; Yin, J.Q. Alu-directed transcriptional regulation of some novel miRNAs. BMC Genom. 2009, 10, 563. [Google Scholar] [CrossRef]
- Lorenzetti, A.P.R.; de Antonio, G.Y.A.; Paschoal, A.R.; Domingues, D.S. Plant TE-MIR DB: A database for transposable element-related microRNAss in plant genomes. Funct. Integr. Genom. 2016, 16, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Qin, S.; Li, W.; Chen, L.; Ma, F. MDTE DB: A database for microRNAs derived from Transposable element. IEEE/ACM Trans. Comput. Biol. Bioinform. 2016, 13, 1155–1160. [Google Scholar] [CrossRef]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Morgado, L.; Johannes, F. Computational tools for plant small RNA detection and categorization. Brief. Bioinform. 2019, 20, 1181–1192. [Google Scholar] [CrossRef]
- Ozata, D.M.; Gainetdinov, I.; Zhoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Ryazansky, S.S. Biogenesis, evolution, and functions of plant microRNAs. Biochemistry 2013, 78, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Xia, J.; Jin, Y. Domestication of transposable elements into microRNA genes in plants. PLoS ONE 2011, 6, e19212. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, B.; Li, W.; Qin, W.; Huang, B.; Jin, Y. Cloning of novel repeat-associated small RNAs derived from hairpin precursors in Oryza sativa. Acta Biochim. Biophys. Sin. 2007, 39, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Piriyapongsa, J.; Jordan, I.K. Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 2008, 14, 814–821. [Google Scholar] [CrossRef]
- Kubiak, M.R.; Makalowska, I. Protein-coding genes’ retrocopies and their functions. Viruses 2017, 9, 80. [Google Scholar] [CrossRef]
- Pedro, D.L.F.; Lorenzetti, A.P.R.; Dominguez, D.S.; Paschoal, A.R. PlaNC-TE: A comprehensive knowledgebase of non-coding RNAs and transposable elements in plants. Database 2018, 2018, bay078. [Google Scholar] [CrossRef]
- Poretti, M.; Praz, C.R.; Meile, L.; Kalin, C.; Schaefer, L.K.; Schlafli, M.; Widrig, V.; Sanchez-Vallet, A.; Wicker, T.; Bourras, S. Domestication of High-Copy Transposons Underlays the Wheat Small RNA Response to an Obligate Pathogen. Mol. Biol. Evol. 2020, 37, 839–848. [Google Scholar] [CrossRef]
- Campo, S.; Sanuy, F.S.; Ramirez, R.C.; Gomez-Ariza, J.; Baldrich, P.; Campos-Soriano, L.; Soto-Suarez, M.; Segundo, B.S. A novel Transposable element-derived microRNA participates in plant immunity to rice blast disease. Plant Biotechnol. J. 2021, 19, 1798–1811. [Google Scholar] [CrossRef]
- Panda, K.; McCue, A.D.; Slotkin, R.K. Arabidopsis RNA Polymerase IV generates 21-22 nucleotide small RNAs that can participate in RNA-directed DNA methylation and may regulate genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190417. [Google Scholar] [CrossRef]
- Marakli, S. In silico determination of transposon-derived miRNAs and targets in Aegilops species. J. Biomol. Dyn. 2020, 38, 3098–3109. [Google Scholar] [CrossRef]
- Spengler, R.M.; Oakley, C.K.; Davidson, B.L. Functional microRNAs and target sites are created by lineage-specific transposition. Hum. Mol. Genet. 2014, 23, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Smalheiser, N.R.; Torvik, V.I. Mammalian microRNAs derived form genomic repeats. Trends Genet. 2005, 21, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Borchert, G.M.; Lanier, W.; Davidson, B.L. RNA polylmerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 2006, 13, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Piriyapongsa, J.; Marino-Ramirez, L.; Jordan, I.K. Origin and evolution of human microRNAs from transposable elements. Genetics 2007, 176, 1323–1337. [Google Scholar] [CrossRef]
- Devor, E.J.; Peek, A.S.; Lanier, W.; Samollow, P.B. Marsupial-specific microRNAs evolved from marsupial-specific transposable elements. Gene 2009, 448, 187–191. [Google Scholar] [CrossRef]
- Shao, P.; Liao, J.Y.; Guan, D.G.; Yang, J.H.; Zheng, L.; Jing, Q.; Zhou, H.; Qu, L. Drastic expression change of transposon-derived piRNA-like RNAs and microRNAs in early stages of chicken embryos implies a role in gastrulation. RNA Biol. 2012, 9, 212–227. [Google Scholar] [CrossRef]
- Yuan, Z.; Sun, X.; Jiang, D.; Ding, Y.; Lu, Z.; Gong, L.; Liu, H.; Xie, J. Origin and evolution of a placental-specific microRNA family in the human genome. BMC Evol. Biol. 2010, 10, 346–358. [Google Scholar] [CrossRef]
- Yuan, Z.; Sun, X.; Liu, H.; Xie, J. MicroRNA genes derived from repetitive elements and expanded by segmental duplication events in mammalian genomes. PLoS ONE 2011, 6, e17666. [Google Scholar] [CrossRef]
- Borchert, G.M.; Holton, N.W.; Williams, J.D.; Hernan, W.L.; Bishop, I.P.; Dembosky, J.A.; Elste, J.E.; Gregoire, N.S.; Kim, J.A.; Koehler, W.W.; et al. Comprehensive analysis of microRNA genomic loci identifies pervasive repetitive-element origins. Mob. Genet. Elem. 2011, 1, 8–17. [Google Scholar] [CrossRef]
- Filshtein, T.J.; Mackenzie, C.O.; Dale, M.D.; Dela-Cruz, P.S.; Ernst, D.M.; Tiwari, K.B.; Rubin, D.A.; Borchert, G.M.; Larson, E.D. Orbid: Origin-based identification of microRNA targets. Mob. Genet. Elem. 2012, 2, 184–192. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, Q.; Yu, C.; Wang, X.; Hu, S.; Yu, J.; Yu, X. Transposable-element associated small RNAs in Bombyx mori genome. PLoS ONE 2012, 7, e36599. [Google Scholar] [CrossRef] [PubMed]
- Tempel, S.; Pollet, N.; Tahi, F. NcRNAclassifier: A tool for detection and classification of transposable element sequences in RNA hairpins. BMC Bioinform. 2012, 13, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.T.; Cooper, E.A.; Favreau, C.J.; Howell, J.S.; Lane, L.G.; Mills, J.E.; Newman, D.C.; Perry, T.J.; Russell, M.E.; Wallace, B.M.; et al. Continuing analysis of microRNA origins: Formation from transposable element insertions and noncoding RNA mutations. Mob. Genet. Elem. 2013, 3, e27755. [Google Scholar] [CrossRef]
- Gim, J.; Ha, H.; Ahn, K.; Kim, D.; Kim, H. Genome-Wide Identification and Classification of microRNAs derived from repetitive elements. Genom. Inf. 2014, 12, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.N.; Vandeweqe, M.W.; Kern, C.; Schmidt, C.J.; Hoffmann, F.G.; Ray, D.A. Large number of novel miRNAs originate from DNA transposons and are coincident with a large species radiation in bats. Mol. Biol. Evol. 2014, 31, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Jin, P.; Zhou, X.; Chen, L.; Ma, F. The Role of Transposable Elements in the Origin and Evolution of MicroRNAs in Human. PLoS ONE 2015, 10, e0131365. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.T.; Cardin, S.E.; Borcehrt, G.M. Burgeoning evidence indicates that microRNAs were initially formed from transposable element sequences. Mob. Genet. Elem. 2014, 4, e29255. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Huh, J.W.; Kim, H.S. Bioinformatics Analysis of Evolution and Human Disease Related Transposable Element-Derived microRNAs. Life 2020, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.W.; Chen, Y.; Chen, S.; Wang, X. OncomiR: And online resource for exploring pan-cancer microRNA dysregulation. Bioinformatics 2018, 34, 713–715. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H., 3rd; De, S.; Ejogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evas, M.K. Age-related changes in microRNA levels in serum. Aging (Albany NY) 2013, 5, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Sataranatarajan, K.; Feliers, D.; Mariappan, M.M.; Lee, H.J.; Lee, M.J.; Day, R.T.; Yalamanchili, H.B.; Choudhury, G.G.; Barnes, J.L.; Remmen, H.V.; et al. Molecular events in matrix protein metabolism in the aging kidney. Aging Cell 2012, 11, 1065–1073. [Google Scholar] [CrossRef]
- Smith-Vikos, T.; Liu, Z.; Parsons, C.; Gorospe, M.; Ferrucci, L.; Gill, T.M.; Slack, F.J. A serum miRNA profile of human longevity: Findings from the Baltimore Longitudinal Study of Aging (BLSA). Aging (Albany NY) 2016, 8, 2971–2987. [Google Scholar] [CrossRef]
- Zhang, T.; Brinkley, T.E.; Liu, K.; Feng, X.; Marsh, A.P.; Kritchevsky, S.; Zhou, X.; Nicklas, B.J. Circulating miRNAs as biomarkers of gait speed responses to aerobic exercise training in obese older adults. Aging (Albany NY) 2017, 9, 900–913. [Google Scholar] [CrossRef]
- Dellago, H.; Preschitz-Kammerhofer, B.; Terlecki-Zaniewicz, L.; Schreiner, C.; Grillari, J.; Wieser, M. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell 2013, 12, 446–458. [Google Scholar] [CrossRef]
- Cho, J.H.; Dimri, M.; Dimri, G.P. MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence. J. Biol. Chem. 2015, 290, 10555–10567. [Google Scholar] [CrossRef]
- Raihan, O.; Brishti, A.; Molla, M.R.; Li, W.; Zhang, Q.; Xu, P.; Khan, M.I.; Zhang, J.; Liu, Q. The Age-dependent Elevation of miR-335-3p Leads to Reduced Cholesterol and Impaired Memory in Brain. Neuroscience 2018, 390, 160–173. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Zhang, C.; Jing, Y.; Wang, C.; Liu, C.; Zhang, R.; Wang, J.; Zhang, J.; Zen, K.; et al. Investigation of microRNA expression in human serum during the aging process. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 102–109. [Google Scholar] [CrossRef]
- Guo, D.; Ye, Y.; Qi, J.; Tan, X.; Zhang, Y.; Ma, Y.; Li, Y. Age and sex differences in microRNAs expression during the process of thymus aging. Acta Biochim. Biophys. Sin. 2017, 49, 409–419. [Google Scholar] [CrossRef]
- Nidadavolu, L.S.; Niedernhofer, L.J.; Khan, S.A. Identification of microRNAs dysregulated in cellular senescence driven by endogenous genotoxic stress. Aging (Albany NY) 2013, 5, 460–473. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B. A newly identified lncRNA MaR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Liu, D.; Zhao, J.; Xu, J.; Ren, J.; Hu, Y.; Wang, Z.; Hou, Y.; Zhao, G. MiR-495 Promotes Senescence of Mesenchymal Stem Cells by Targeting Bmi-1. Cell Physiol. Biochem. 2017, 42, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sabbagh, J.J.; Blair, L.J.; Darling, A.L.; Wen, X.; Dickey, C.A. MicroRNA-511 Binds to FKBP5 mRNA, Which Encodes a Chaperone Protein, and Regulates Neuronal Differentiation. J. Biol. Chem. 2016, 291, 17897–17906. [Google Scholar] [CrossRef] [PubMed]
- Ipson, B.R.; Fletcher, M.B.; Espinoza, S.E.; Fisher, A.L. Identifying Exosome-Derived MicroRNAs as Candidate Biomarkers of Frailty. J. Frailty Aging 2018, 7, 100–103. [Google Scholar] [CrossRef]
- Dluzen, D.F.; Kim, Y.; Bastian, P.; Zhang, Y.; Lehrmann, E.; Becker, K.G.; Hooten, N.N.; Evas, M.K. MicroRNAs Modulate Oxidative Stress in Hypertension through PARP-1 Regulation. Oxid. Med. Cell. Longev. 2017, 2017, 3984280. [Google Scholar] [CrossRef]
- Behbahanipour, M.; Peymani, M.; Salari, M.; Hashemi, M.; Nasr-Esfahani, M.H.; Ghaedi, K. Expression Profiling of Blood microRNAs 885, 361, and 17 in the Patients with the Parkinson’s disease: Integrating Interatction Data to Uncover the Possible Triggering Age-Related Mechanisms. Sci. Rep. 2019, 9, 13759. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Buric, I.; George-Pandeth, A.; Flurkey, K.; Harrison, D.E.; Yuan, R.; Peters, L.L.; Kuchel, G.A.; Melzer, D.; Harries, L.W. MicroRNAs miR-203-3p, miR-664-3p and miR-708-5p are associated with median strain lifespan in mice. Sci. Rep. 2017, 7, 44620. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Qu, Y.; Jiang, W.; Lv, C. Pharmacodynamic and pharmacokinetic assessment of pulmonary rehabilitation mixture for the treatment of pulmonary fibrosis. Sci. Rep. 2017, 7, 3458. [Google Scholar] [CrossRef]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef]
- Wyman, A.E.; Noor, Z.; Fishelevich, R.; Lockatell, V.; Shah, N.G.; Todd, N.W.; Atamas, S.P. Sirtuin 7 is decreased in pulmonary fibrosis and regulates the fibrotic phenotype of lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L945–L958. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, L.; Wang, W.; Wang, J.; Wang, J.; Xu, Z. Discovery and validation of extracellular/ circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene 2015, 562, 138–144. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, X.; Yang, Y.; Mishra, A.; Liang, Y.; Zeng, X.; Yang, X.; Xu, D.; Blackburn, M.R.; Henke, C.A.; et al. MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast proliferation and activation. J. Biol. Chem. 2017, 292, 16420–16439. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Song, X.; Sun, W.; Zhang, J.; Liu, Y.; Li, H.; Meng, C.; Zhang, J.; Zheng, Q.; et al. Potential regulatory role of circular RNA in idiopathic pulmonary fibrosis. Int. J. Mol. Med. 2018, 42, 3256–3268. [Google Scholar] [CrossRef]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Guo, Y.F.; Yang, S.M.; Li, J. MiR-340-5p mitigates the proliferation and activation of fibroblast in lung fibrosis by targeting TGF-β/p38/ATF1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6252–6261. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Gu, L.N.; Qi, J.; Xia, Q.; Tian, L.; Jiang, W.; Cao, M. Construciton of potential idiopathic pulmonary fibrosis related microRNA and messenger RNA regulatory network. Chin. Med. J. 2021, 134, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Y.; Yu, H.; Machan, J.T.; Alladin, A.; Ramirez, J.; Taliano, R.; Hart, J.; Chen, Q.; Terek, R.M. Anti-miRNA Oligonucleotide Therapy for Chondrosarcoma. Mol. Cancer Ther. 2019, 18, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xiong, G.; Guo, S.; Xu, C.; Xu, R.; Guo, P.; Shu, D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, G.; Kong, D. miRNA-27a promotes the proliferation and inhibits apoptosis of human pancreatic cancer cells by Wnt/β-catenin pathway. Oncol. Rep. 2018, 39, 755–763. [Google Scholar] [CrossRef]
- Han, S.; Li, G.; Jia, M.; Zhao, Y.; He, C.; Huang, M.; Jiang, L.; Wu, M.; Yang, J.; Ji, X.; et al. Delivery of Anti-miRNA-221 for Colorectal Carcinoma Therapy Using Modified Cord Blood Mesenchymal Stem Cells-Derived Exosomes. Front. Mol. Biosci. 2021, 20, 8, 743013. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Morsalin, S.; Rao, V.N.; Reddy, E.S.P. Decoding of Non-Coding DNA and Non-Coding RNA: Pri-Micro RNA-Encoded Novel Peptides Regulate Migration of Cancer Cells. J. Pharm. Sci. Pharmacol. 2017, 3, 23–27. [Google Scholar] [CrossRef]
- Niu, L.; Lou, F.; Sun, Y.; Sun, L.; Cai, X.; Liu, Z.; Zhou, H.; Wang, H.; Wang, Z.; Bai, J.; et al. A micropeptide encoded by lncRNA MIR155HG suppresses autoimmune inflammation via modulating antigen presentation. Sci. Adv. 2020, 6, eaaz2059. [Google Scholar] [CrossRef]
- Kang, M.; Tang, B.; Li, J.; Zhou, Z.; Liu, K.; Wang, R.; Jiang, Z.; Bi, F.; Patrick, D.; Kim, D.; et al. Identification of miPEP133 as a novel tumor-suppressor microprotein encoded by miR-34a pri-miRNA. Mol. Cancer 2020, 19, 143. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A.; Mita, P.; McKerrow, W.; Fenyo, D.; Boeke, J.D.; Linker, S.B.; Gage, F.H.; Kreiling, J.A.; Petrashen, A.P.; et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature 2021, 596, 43–53. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Jansz, N.; Faulkner, G.J. Endogenous retroviruses in the origins and treatment of cancer. Genome Biol. 2021, 22, 147. [Google Scholar] [CrossRef]
- Razooky, B.S.; Obermayer, B.; O’May, J.B.; Tarakhovsky, A. Viral Infection Identifies Micropeptides Differentially Regulated in smORF-Containing lncRNAs. Genes 2017, 8, 206. [Google Scholar] [CrossRef]
- Prel, A.; Dozier, C.; Combier, J.P.; Plaza, S.; Besson, A. Evidence That Regulation of Pri-miRNA/miRNA Expression Is Not a General Rule of miPEPs Function in Humans. Int. J. Mol. Sci. 2021, 22, 3432. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, S.; Wang, Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- McJunkin, K. Maternal effects of microRNAs in early embryogenesis. RNA Biol. 2018, 15, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Muraoka, M.; Okada, H.; Toyoda, A.; Ajima, R.; Saga, Y. The RNA helicase DDX6 controls early mouse embryogenesis by repressing aberrant inhibition of BMP signaling through miRNA-mediated gene silencing. PLoS Genet. 2022, 18, e1009967. [Google Scholar] [CrossRef]
- Wei, W.; Morrish, T.A.; Alisch, R.S. A transient assay reveals that cultured human cells can accommodate multiple LINE-1 retrotransposition events. Anal. Biochem. 2000, 284, 435–438. [Google Scholar] [CrossRef]
- Garcia-Perez, J.L.; Marchetto, M.C.; Muotri, A.R.; Coufal, N.G.; Gage, F.H.; O’Shea, K.S.; Moran, J.V. LINE-1 retrotransposition in human embryonic stem cells. Hum. Mol. Genet. 2007, 16, 1569–1577. [Google Scholar] [CrossRef]
- Macia, A.; Munoz-Lopez, M.; Cortes, J.L.; Hastings, R.K.; Morell, S.; Lucena-Aguilar, G.; Marchal, J.A.; Badge, R.M.; Garcia-Perez, J.L. Epigenetic control of retrotransposons expression in human embryonic stem cells. Mol. Cell. Biol. 2011, 31, 300–316. [Google Scholar] [CrossRef]
- Prak, E.T.; Dodson, A.W.; Farkash, E.A.; Kazazian, H.H., Jr. Tracking an embryonic L1 retrotransposition event. Proc. Nat. Acad. Sci. USA 2003, 100, 1832–1837. [Google Scholar] [CrossRef]
- Lee, K.H.; Chiu, S.; Lee, Y.K.; Greenhalgh, D.G.; Cho, K. Age-dependent and tissue-specific structural changes in the C57BL/6J mouse genome. Exp. Mol. Pathol. 2012, 93, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jachowicz, J.W.; Bing, X.; Pontabry, J.; Boskovic, A.; Rando, O.J.; Torres-Padilla, M. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nat. Genet. 2017, 49, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Honson, D.D.; Macfarlan, T.S. A lncRNA-like Role for LINE1s in Development. Dev. Cell 2018, 46, 132–134. [Google Scholar] [CrossRef] [PubMed]
| № | miRNA Designation | Transposon, miRNA Source [Author] | Diseases and Changes (Increased—↑; Decreased—↓) in miRNA Expression (Expressed Tissue) [Author] | miRNA Expression in Aging (Increased—↑; Decreased—↓) (Expressed Tissue) [Author] |
|---|---|---|---|---|
| (1) | miR-1183 | LINE/L2 [37,41] | ||
| (2) | miR-1200 | SINE/MIR [33,37,41] | ||
| (3) | miR-1202 | LTR/ERV1 [37,41]; LTR/MER52A [43] | ||
| (4) | miR-1205 | SINE/MIR [33,37] | ||
| (5) | miR-1246 | LTR/ERVL-MaLR [37,41] | ||
| (6) | miR-1249 | LINE/L2 [35,37,41] | ↑: BLCA, HNSC, KIRC, LUSC, PRAD, STAD, UCEC; ↓: BRCA, COAD, READ, THCA (tumor tissue) [44] | |
| (7) | miR-1254-1 | SINE/Alu [37,41] | ||
| (8) | miR-1254-2 | |||
| (9) | miR-1255a | DNA-TE/TcMar-Tigger [37,41] | ||
| (10) | miR-1255b-1 | |||
| (11) | miR-1255b-2 | |||
| (12) | miR-1256 | |||
| (13) | miR-1257 | LTR/ERVL-MaLR [37,41] | ||
| (14) | miR-1260b | DNA-TE/TcMar-Tigger [37] | ||
| (15) | miR-1261 | DNA-TE/TcMar-Tigger [37,41,43] | ||
| (16) | miR-1263 | LTR/ERV1 [37,41] | ||
| (17) | miR-1264 | LINE/L2 [33,37,41] | ||
| (18) | miR-1266 | SINE/MIR [37,41] | ↑: BLCA, BRCA, CHOL, ESCA, KICH, KIRC, KIRP, LIHC, PRAD, STAD, UCEC; ↓: COAD (tumor tissue) [44] | |
| (19) | miR-1267 | LTR/ERVL-MaLR [37,41] | ||
| (20) | miR-1268a | SINE/Alu [37,41,43] | ||
| (21) | miR-1268b | |||
| (22) | miR-1269a | LTR/ERVL [35,37,41] | ↑: BLCA, BRCA, HNSC, LIHC, LUAD, LUSC, PRAD, STAD, THCA, UCEC; ↓: CHOL, KICH (tumor tissue) [44] | |
| (23) | miR-1269b | LTR/ERVL [37,41] | ||
| (24) | miR-1271 | LINE/L2 [35,37,41] | ↑: BLCA, ESCA, KIRC, LUSC; ↓: BRCA, COAD, KICH, LIHC, LUSC (tumor tissue) [44] | |
| (25) | miR-1273a | SINE/Alu [37,41,43] | ||
| (26) | miR-1273c | |||
| (27) | miR-1273d | |||
| (28) | miR-1273f | |||
| (29) | miR-1273g | |||
| (30) | miR-1273h | |||
| (31) | miR-1285-1 | |||
| (32) | miR-1285-2 | |||
| (33) | miR-1289-1 | DNA-TE/hAT-Charlie [37,41] | ||
| (34) | miR-1289-2 | |||
| (35) | miR-1290 | DNA-TE/TcMar-Tigger [37,41] | ||
| (36) | miR-1293 | SINE/Alu [33,37,41] | ||
| (37) | miR-1296 | LINE/L2 [12] | ↑: BLCA, ESCA, LUSC, PRAD, UCEC; ↓: BRCA, COAD, KIRC, LIHC, READ, THCA (tumor tissue) [44] | |
| (38) | miR-1298 | DNA-TE/X24 [41] | ||
| (39) | miR-130a | LINE/L2 [43] | ||
| (40) | miR-130b | SINE/MIR [29] | ||
| (41) | miR-1302-1 | DNA/hAT [32,37,39,41,43] | ||
| (42) | miR-1302-10 | DNA/hAT [32,37,39,41] | ||
| (43) | miR-1302-11 | |||
| (44) | miR-1302-2 | |||
| (45) | miR-1302-3 | |||
| (46) | miR-1302-4 | |||
| (47) | miR-1302-5 | |||
| (48) | miR-1302-6 | |||
| (49) | miR-1302-7 | |||
| (50) | miR-1302-8 | |||
| (51) | miR-1302-9 | |||
| (52) | miR-1303 | SINE/Alu [37,41,43] | ||
| (53) | miR-1304 | |||
| (54) | miR-1321 | SINE/MIR [37,41] | ||
| (55) | miR-1343 | LINE/L2 [12] | ↓: IPF (lung tissue) [67] | |
| (56) | miR-151a | LINE/L2 [29,35,37,43] | ↑: BLCA, BRCA, CESC, COAD, ESCA, HNSC, KICH, KIRC, KIRP, LIHC, LUAD, LUSC, PRAD, READ, STAD, UCEC (tumor tissue) [44] | ↓ (serum) [46] |
| (57) | miR-151b | LINE/L2 [37,41,43] | ||
| (58) | miR-1587 | LTR/ERVL-MaLR [37,41,43] | ||
| (59) | miR-1825 | LINE/L2 [37,41] | ||
| (60) | miR-1911 | LTR/Gypsy [33,37,41] | ↑: ESCA, HNSC, LUSC, STAD (tumor tissue) [44] | |
| (61) | miR-192 | LINE/L2 [12] | ↑: BLCA, BRCA, COAD, KIRC, LUAD, LUSC, PRAD, READ, STAD, UCEC; ↓: CHOL, KICH, KIRP, LICH, THCA (tumor tissue) [44] | ↑ (kidney tissue) [47] |
| (62) | miR-1972-1 | SINE/Alu [37,41,43] | ||
| (63) | miR-1972-2 | |||
| (64) | miR-2054 | DNA-TE/Helitron [37] | ||
| (65) | miR-211 | LINE/L2 [12] | ↑: KIRC, KIRP, LIHC; ↓: BRCA, HNSC, LUAD (tumor tissue) [44] | ↑ (serum) [48] |
| (66) | miR-2114 | LINE/CR1 [37] | ↑: BRCA, KIRC, LIHC (tumor tissue) [44] | |
| (67) | miR-2115 | LINE/L1 [37,41] | ↑: BRCA (tumor tissue) [44] | |
| (68) | miR-219-1 | LTR/Gypsy [37] | ||
| (69) | miR-224 | DNA-TE/MER135 [37,41,43] | ↑: CESC, ESCA, HNSC, KIRC, LIHC, LUAD, LUSC, UCEC; ↓: BRCA, KICH (tumor tissue) [44] | |
| (70) | miR-23c | SINE/tRNA [37] | ||
| (71) | miR-2355 | LINE/RTE-BovB [37,41,43] | ↑: BLCA, COAD, ESCA, HNSC, KICH, KIRC, KIRP, LIHC, LUAD, LUSC, PRAD, READ, STAD, UCEC; ↓: LIHC, PAAD, THCA (tumor tissue) [44] | |
| (72) | miR-28 | LINE/L2 [29,35,37,43] | ↑: HNSC, KIRC, LUAD, LUSC, PRAD; ↓: BRCA, CHOL, COAD, ESCA, PCPG, READ, STAD, THCA (tumor tissue) [44] | ↓ (blood) [49] |
| (73) | miR-2909 | LTR/ERVL-MaLR [37,41] | ||
| (74) | miR-302e | SINE/MIR [37,41,43] | ||
| (75) | miR-31 | LINE/L2 [12] | ↑: BLCA, CESC, HNSC, KIRP, LUAD, LUSC, STAD, THCA, UCEC (tumor tissue) [44]; IPF (lung tissue) [66]; ↓: KICH, KIRC, PRAD (tumor tissue) [44] | ↑ endothelial cells [50], (breast tissue) [51] |
| (76) | miR-302 | SINE/MIR [41] | ↑: IPF (lung tissue) [66] | |
| (77) | miR-3116-1 | LINE/L2 [37,41] | ||
| (78) | miR-3116-2 | |||
| (79) | miR-3118-1 | LINE/L1 [37,41,43] | ||
| (80) | miR-3118-2 | |||
| (81) | miR-3118-3 | |||
| (82) | miR-3118-4 | |||
| (83) | miR-3118-5 | |||
| (84) | miR-3118-6 | |||
| (85) | miR-3133 | |||
| (86) | miR-3134 | |||
| (87) | miR-3135a | SINE/Alu [37,41] | ||
| (88) | miR-3135b | |||
| (89) | miR-3137 | DNA-TE/TcMar-Tigger [37,41] | ||
| (90) | miR-3139 | LINE/L2 [37,41] | ||
| (91) | miR-3144 | LINE/L1 [37,41,43] | ↑: HNSC, KICH (tumor tissue) [44] | |
| (92) | miR-3149 | LINE/L1 [41] | ||
| (93) | miR-3159 | SINE/Alu [37,41] | ||
| (94) | miR-3161 | DNA-TE/hAT [37] | ||
| (95) | miR-3163 | SINE/MIR [37,41] | ||
| (96) | miR-3164 | DNA-TE/ TcMar-Tigger [41] | ||
| (97) | miR-3166 | LINE/L2 [37,41] | ||
| (98) | miR-3168 | SINE/MIR [37,41] | ||
| (99) | miR-3169 | |||
| (100) | miR-3174 | DNA-TE/hAT-Charlie [37,41] | ||
| (101) | miR-3176 | LINE/L1 [37] | ||
| (102) | miR-3179-1 | SINE/Alu [33,37,41] | ||
| (103) | miR-3179-2 | SINE/Alu [33,37,41] | ||
| (104) | miR-3179-3 | |||
| (105) | miR-3185 | DNA-TE/hAT [37] | ||
| (106) | miR-3194 | SINE/MIR [37,41] | ↑: STAD (tumor tissue) [44] | |
| (107) | miR-3200 | ERV-L [35,37,41] | ↑: BLCA, BRCA, CHOL, HNSC, KIRP, LIHC, LUSC, STAD, UCEC; ↓: KIRC (tumor tissue) [44] | |
| (108) | miR-3201 | LINE/L2 [37,41] | ||
| (109) | miR-320b-2 | LINE/L2 [33,37,41] | ||
| (110) | miR-320c-1 | LINE/RTE [37] | ||
| (111) | miR-320c-2 | DNA-TE/Ginger2/TDD [37] | ||
| (112) | miR-320d-1 | LINE/L1 [43] | ||
| (113) | miR-320d-2 | LINE/CR1 [37] | ||
| (114) | miR-325 | LINE/L2 [29,37,39,41] | ||
| (115) | miR-326 | DNA-TE/hAT-Tip100 [37,41] | ↑: BLCA, KIRC, PCPG, UCEC; ↓: BRCA, COAD, KICH, LIHC, LUSC, READ, THCA (tumor tissue) [44]; IPF (lung tissue) [67] | |
| (116) | miR-330 | SINE/MIR [29,37] | ||
| (117) | miR-335 | SINE/MIR [33,37,41,43] | ↑: BLCA, COAD, ESCA, HNSC, LUAD, LUSC, PRAD, STAD, THCA, UCEC; ↓: BRCA, KICH, KIRC, LIHC (tumor tissue) [44]; IPF (lung tissue) [66] | ↑ (brain tissue) [52] |
| (118) | miR-340 | DNA-TE/TcMar [35,37,41,43] | ↑: BRCA, COAD, KICH, KIRC, KIRP, LUAD, LUSC, PRAD, UCEC (tumor tissue) [44]; IPF (lung tissue) [70] ↓: CHOL, LIHC, PAAD (tumor tissue) [44] | ↑ (serum) [53] |
| (119) | miR-342 | SINE/tRNA-RTE [35,37,41,43] | ↑: BLCA, BRCA, CESC, HNSC, KIRC, KIRP, PRAD, STAD, UCEC; ↓: COAD, LIHC, LUAD, PAAD, READ, THCA (tumor tissue) [44] | |
| (120) | miR-345 | SINE/MIR [29,37] | ||
| (121) | miR-361 | DNA-TE/hAT [37] | ||
| (122) | miR-3611 | DNA/TcMar-Tigger [37,41] | ||
| (123) | miR-3617 | SINE/MIR [37,41] | ||
| (124) | miR-3622a | SINE/Alu [12] | ↓: LUAD (tumor tissue) [44] | |
| (125) | miR-3622b | SINE/Alu [37,41] | ||
| (126) | miR-3646 | SINE/MIR [33,37] | ||
| (127) | miR-3648 | DNA-TE/MER [37] | ||
| (128) | miR-3657 | LINE/L1 [37,41] | ||
| (129) | miR-3664 | DNA-TE/TcMar-Tigger [33,37,41] | ||
| (130) | miR-3665 | DNA-TE/EnSpm [37] | ||
| (131) | miR-3667 | LTR/ERVL-MaLR [37,41] | ||
| (132) | miR-3668 | SINE/MIR [33,37] | ||
| (133) | miR-3670-1 | LTR/ERVL [37,41] | ||
| (134) | miR-3670-2 | |||
| (135) | miR-3672 | LINE/L1 [37,41] | ||
| (136) | miR-3674 | LTR/ERV1 [37,41] | ||
| (137) | miR-3680-1 | DNA-TE/hAT-Tip100 [41] | ||
| (138) | miR-3680-2 | |||
| (139) | miR-3681 | LTR/ERVL [37,41] | ||
| (140) | miR-3686 | LINE/L1 [37,41] | ||
| (141) | miR-3689c | LINE/L1 [37] | ||
| (142) | miR-370 | SINE/MIR [29,37] | ||
| (143) | miR-3713 | DNA/TcMar-Tigger [37,41] | ||
| (144) | miR-374a | LINE/L2 [37,41,43] | ↑: BLCA, BRCA, COAD, KIRC, KIRP, PRAD, READ, STAD; ↓: CHOL, HNSC, LUSC (tumor tissue) [44]; ↓: IPF (lung tissue) [66] | |
| (145) | miR-374b | LINE/L2 [29,37] | ↑: BLCA, BRCA, COAD, ESCA, HNSC, KIRC, KIRP, PRAD, STAD, UCEC; ↓: THCA (tumor tissue) [44] | |
| (146) | miR-374c | LINE/L2 [37,41] | ||
| (147) | miR-378a | SINE/MIR [29,37,43] | ↑: PAAD; ↓: BRCA, CHOL, COAD, HNSC, LIHC, LUAD, PAAD, PRAD, READ, STAD (tumor tissue) [44] | ↑ (thymus tissue) [54] |
| (148) | miR-378b | SINE/MIR [37,41,43] | ||
| (149) | miR-378d-1 | |||
| (150) | miR-378d-2 | |||
| (151) | miR-378e | |||
| (152) | miR-378f | |||
| (153) | miR-378g | |||
| (154) | miR-378h | |||
| (155) | miR-378i | SINE/MIR [37,43] | ||
| (156) | miR-3908 | SINE/Alu [37,41] | ||
| (157) | miR-3909 | LINE/L2 [12,37] | ↑: LIHC (tumor tissue) [44] | |
| (158) | miR-3910-1 | LINE/L1 [37,41] | ||
| (159) | miR-3910-2 | |||
| (160) | miR-3912 | |||
| (161) | miR-3915 | |||
| (162) | miR-3919 | LINE/L1 [37] | ||
| (163) | miR-3920 | LINE/L2 [33,37,41] | ||
| (164) | miR-3921 | LINE/L2 [33,37] | ||
| (165) | miR-3923 | LTR/ERVL-MaLR [37,41] | ||
| (166) | miR-3925 | DNA-TE/TcMar-Tigger [37,41] | ||
| (167) | miR-3927 | LTR/ERVL-MaLR [37,41] | ||
| (168) | miR-3929 | SINE/Alu [37,41] | ||
| (169) | miR-3934 | SINE/MIR [35,37,41] | ↑: BRCA, HNSC, KIRC, LUSC, STAD, UCEC (tumor tissue) [44] | |
| (170) | miR-3936 | LTR/ERVL [33,35,37,41] | ||
| (171) | miR-3937 | LTR/ERV3 [37] | ||
| (172) | miR-3972 | DNA-TE/hAT-Tip100 [37,41] | ||
| (173) | miR-3973 | DNA-TE/TcMar-Tigger [41] | ||
| (174) | miR-3977 | DNA-TE/hAT-Tip100 [41] | ||
| (175) | miR-421 | LINE/L2 [29,35,37,43] | ↑: BLCA, BRCA, ESCA, HNSC, KIRP, LIHC, LUAD, LUSC, STAD, UCEC; ↓: THCA (tumor tissue) [44] | |
| (176) | miR-422a | SINE/MIR [37,41] | ||
| (177) | miR-4263 | LINE/L1 [37,41] | ||
| (178) | miR-4281 | LTR/ERV1 [37] | ||
| (179) | miR-4286 | LTR/ERV3 [37] | ||
| (180) | miR-4288 | LTR/ERVL [33,37,41] | ||
| (181) | miR-4293 | SINE/tRNA [37,41] | ||
| (182) | miR-4311 | LINE/L2 [41] | ||
| (183) | miR-4317 | SINE/MIR [33,37] | ||
| (184) | miR-4418 | LINE/L2 [37,41] | ||
| (185) | miR-4419a | SINE/Alu [37,41] | ||
| (186) | miR-4419b | |||
| (187) | miR-4420 | SINE/MIR [37,41] | ||
| (188) | miR-4421 | LTR/ERV1 [37,41] | ||
| (189) | miR-4422 | LTR/Gypsy [37,41] | ||
| (190) | miR-4424 | LINE/L1 [37,41] | ||
| (191) | miR-4425 | SINE/MIR [37,41] | ||
| (192) | miR-4428 | LTR/ERV1 [37,41] | ||
| (193) | miR-4430 | SINE/Alu [37,41] | ||
| (194) | miR-4431 | LTR/ERVL-MaLR [37,41] | ||
| (195) | miR-4433 | LINE/L2 [37,41] | ||
| (196) | miR-4433b | |||
| (197) | miR-4438 | LINE/L1 [37,41] | ||
| (198) | miR-4445 | |||
| (199) | miR-4447 | DNA-TE/hAT-Charlie [37,41] | ||
| (200) | miR-4448 | LTR/ERVK [37,41] | ||
| (201) | miR-4452 | SINE/Alu [37,41,43] | ||
| (202) | miR-4454 | LTR/ERV1 [37,41] | ||
| (203) | miR-4455 | LINE/L1 [37,41] | ||
| (204) | miR-4457 | |||
| (205) | miR-4459 | SINE/Alu [37,41] | ||
| (206) | miR-4460 | LTR/ERVL [37,41] | ||
| (207) | miR-4463 | DNA-TE/hAT-Charlie [37,41] | ||
| (208) | miR-4468 | LINE/R1 [37] | ||
| (209) | miR-4472-2 | SINE/Alu [37,41] | ||
| (210) | miR-4477a | DNA-TE/TcMar-Tigger [37,41] | ||
| (211) | miR-4477b | |||
| (212) | miR-4480 | SINE/MIR [37,41] | ||
| (213) | miR-4481 | LTR/ERVL-MaLR [37,41] | ||
| (214) | miR-4483 | SINE/Alu [37,41] | ||
| (215) | miR-4484 | LTR/ERV1 [37,41] | ||
| (216) | miR-4491 | DNA-TE/hAT-Blackjack [37,41] | ||
| (217) | miR-4494 | DNA-TE/TcMar-Tigger [37,41] | ||
| (218) | miR-4495 | SINE/MIR [37,41] | ||
| (219) | miR-4496 | |||
| (220) | miR-450b | LINE/L1 [33,35,37,41,43] | ↑: BLCA, BRCA, COAD, ESCA, HNSC, KIRC, LUAD, LUSC, READ, STAD, THCA; ↓: CHOL, KICH, KIRP, LIHC, PRAD, UCEC (tumor tissue) [44] | ↓ (liver tissue) [55] |
| (221) | miR-4501 | SINE/MIR [37,41] | ||
| (222) | miR-4502 | DNA-TE/TcMar-Tigger [37,41] | ||
| (223) | miR-4504 | LINE/L1 [37,41] | ||
| (224) | miR-4506 | SINE/MIR [37,41] | ||
| (225) | miR-4507 | DNA-TE/P-1 [37] | ||
| (226) | miR-4508 | SINE [37] | ||
| (227) | miR-4510 | LINE/L2 [37,41] | ||
| (228) | miR-4512 | SINE/Alu [37,41] | ||
| (229) | miR-4518 | DNA/hAT-Charlie [37,41] | ||
| (230) | miR-4520a | SINE/MIR [37,41] | ||
| (231) | miR-4520b | SINE/tRNA [37] | ||
| (232) | miR-4525 | LTR/ERV1 [37,41] | ||
| (233) | miR-4527 | LTR/ERVL-MaLR [37,41] | ||
| (234) | miR-4537 | DNA-TE/P-1 [37] | ||
| (235) | miR-4538 | DNA-TE/P-1 [37] | ||
| (236) | miR-4640 | LTR/Copia [37] | ||
| (237) | miR-4656 | SINE/tRNA [37] | ||
| (238) | miR-466 | LINE/L1 [37,41,43] | ||
| (239) | miR-4661 | LTR/Gypsy [41] | ||
| (240) | miR-4662a | LINE/L1 [37,41] | ||
| (241) | miR-4662b | |||
| (242) | miR-4666b | LTR/ERVL-MaLR [41] | ||
| (243) | miR-4671 | SINE/tRNA-RTE [41] | ||
| (244) | miR-4672 | SINE/MIR [37,41] | ||
| (245) | miR-4676 | LINE/L2 [37,41] | ||
| (246) | miR-4684 | DNA-TE/hAT-Charlie [37,41] | ||
| (247) | miR-4699 | LINE/L2 [37,41] | ||
| (248) | miR-4703 | DNA-TE/TcMar-Tigger [41] | ||
| (249) | miR-4704 | LTR/ERVL-MaLR [37,41] | ||
| (250) | miR-4712 | SINE-MIR [37,41] | ||
| (251) | miR-4731 | LINE/CR1 [37,41] | ||
| (252) | miR-4739 | LTR/Gypsy [37] | ||
| (253) | miR-4750 | LINE/CR1 [37] | ||
| (254) | miR-4753 | LINE/L1 [37,41] | ||
| (255) | miR-4756 | DNA/hAT-Tip100 [37,41] | ||
| (256) | miR-4771-1 | LINE/L1 [37] | ||
| (257) | miR-4771-2 | |||
| (258) | miR-4772 | LINE/L1 [37,41] | ||
| (259) | miR-4775 | DNA-TE/TcMar-Tc1 [41] | ||
| (260) | miR-4786 | LINE/L1 [37,41] | ||
| (261) | miR-4797 | SINE/5S-Deu-L2 [41] | ||
| (262) | miR-4800 | DNA-TE/Sola [37] | ||
| (263) | miR-4801 | SINE/MIR [37,41] | ||
| (264) | miR-487b | SINE/MIR [33,37,41] | ↑: LUAD, LUSC (tumor tissue) [44]; IPF (lung tissue) [71]; ↓: BRCA, HNSC, KICH, KIRC, KIRP, LIHC, PRAD, THCA, UCEC (tumor tissue) [44] | ↑ (skeletal muscles) [56] |
| (265) | miR-493 | LINE/L2 [29,35,37] | ↑: BRCA, ESCA, LUAD, LUSC, READ, STAD (tumor tissue) [44]; IPF (lung tissue) [71] ↓: KICH, KIRC, KIRP, LIHC, PRAD (tumor tissue) [44] | |
| (266) | miR-495 | ERV-L/MaLR [12] | ↑: COAD, LUAD, READ (tumor tissue) [44]; IPF (lung tissue) [71]; ↓: BRCA, HNSC, KICH, KIRC, KIRP, LIHC, THCA, UCEC (tumor tissue) [44] | ↑ (mesenchymal stem cells) [57] |
| (267) | miR-4999 | LINE/L1 [41] | ||
| (268) | miR-5003 | SINE/MIR [41] | ||
| (269) | miR-5007 | LTR/ERVL-MaLR [41] | ||
| (270) | miR-5009 | DNA-TE/TcMar-Tigger [41] | ||
| (271) | miR-5011 | |||
| (272) | miR-502 | LINE/L2 [12] | ↑: BLCA, LIHC, PRAD, STAD, UCEC; ↓: COAD, KIRC, KIRP, LUSC, PAAD, THCA (tumor tissue) [44] | |
| (273) | miR-5094 | SINE/Alu [41] | ||
| (274) | miR-5095 | SINE/Alu [37,41] | ||
| (275) | miR-5096 | |||
| (276) | miR-5100 | SINE/MIR | ||
| (277) | miR-511 | LINE/L1 [12] | ↑: HNSC, PRAD, READ, STAD; ↓: BRCA, CHOL, KICH, KIRP, LIHC, LUSC, PCPG (tumor tissue) [44] | ↑ (brain tissue) [58] |
| (278) | miR-513a-1 | DNA-TE/MER [37,43] | ||
| (279) | miR-513a-2 | |||
| (280) | miR-513b | |||
| (281) | miR-513c | |||
| (282) | miR-517a | SINE/Alu [10] | ↓: LUAD (tumor tissue) [44] | |
| (283) | miR-518d | LINE/RTE [43] | ||
| (284) | miR-520d | SINE/Alu [12] | ↑: LIHC (tumor tissue) [44] | |
| (285) | miR-544a | DNA-TE/hAT-Charlie [37,41,43] | ||
| (286) | miR-544b | |||
| (287) | miR-545 | LINE/L2 [29,35,37,43] | ↑: BRCA, KIRC, LIHC, READ (tumor tissue) [44] | |
| (288) | miR-548a-1 | DNA-TE/TcMar-Mariner [37,41,43] | ||
| (289) | miR-548a-2 | |||
| (290) | miR-548a-3 | |||
| (291) | miR-548aa-1 | |||
| (292) | miR-548aa-2 | |||
| (293) | miR-548ab | |||
| (294) | miR-548ac | |||
| (295) | miR-548ad | |||
| (296) | miR-548ae-1 | |||
| (297) | miR-548ae-2 | |||
| (298) | miR-548ag-1 | |||
| (299) | miR-548ag-2 | |||
| (300) | miR-548ah | |||
| (301) | miR-548ai | |||
| (302) | miR-548aj-1 | |||
| (303) | miR-548aj-2 | |||
| (304) | miR-548ak | |||
| (305) | miR-548al | |||
| (306) | miR-548am | |||
| (307) | miR-548an | |||
| (308) | miR-548ao | |||
| (309) | miR-548ap | |||
| (310) | miR-548aq | |||
| (311) | miR-548ar | |||
| (312) | miR-548as | DNA-TE/TcMar-Mariner [41] | ||
| (313) | miR-548at | |||
| (314) | miR-548au | |||
| (315) | miR-548av | |||
| (316) | miR-548aw | |||
| (317) | miR-548ax | |||
| (318) | miR-548ay | |||
| (319) | miR-548az | |||
| (320) | miR-548b | |||
| (321) | miR-548ba | |||
| (322) | miR-548c | |||
| (323) | miR-548d-1 | |||
| (324) | miR-548d-2 | |||
| (325) | miR-548e | |||
| (326) | miR-548f-1 | |||
| (327) | miR-548f-2 | |||
| (328) | miR-548f-3 | DNA-TE/TcMar-Mariner [41] | ||
| (329) | miR-548f-4 | |||
| (330) | miR-548f-5 | |||
| (331) | miR-548g | |||
| (332) | miR-548h-1 | |||
| (333) | miR-548h-2 | |||
| (334) | miR-548h-3 | |||
| (335) | miR-548h-4 | |||
| (336) | miR-548h-5 | |||
| (337) | miR-548i-1 | |||
| (338) | miR-548i-2 | |||
| (339) | miR-548i-3 | |||
| (340) | miR-548i-4 | |||
| (341) | miR-548j | |||
| (342) | miR-548k | |||
| (343) | miR-548l | |||
| (344) | miR-548m | |||
| (345) | miR-548n | DNA-TE/TcMar-Mariner [39,41] | ||
| (346) | miR-548o | |||
| (347) | miR-548o-2 | |||
| (348) | miR-548p | |||
| (349) | miR-548q | |||
| (350) | miR-548s | |||
| (351) | miR-548t | |||
| (352) | miR-548u | |||
| (353) | miR-548v | |||
| (354) | miR-548w | |||
| (355) | miR-548x | |||
| (356) | miR-548x-2 | |||
| (357) | miR-548y | |||
| (358) | miR-548z | |||
| (359) | miR-549a | SINE/MIR [33,41] | ||
| (360) | miR-551a | LINE/L1 [12] | ↑: BRCA, LUAD, LUSC, STAD (tumor tissue) [44] | |
| (361) | miR-552 | LINE/L1 [29,37,41] | ↑: LIHC, READ, STAD (tumor tissue) [44] | |
| (362) | miR-553 | SINE/MIR [33,41] | ||
| (363) | miR-558 | LTR/ERVL-MaLR [37,39,41] | ||
| (364) | miR-5584 | SINE/MIR [41] | ||
| (365) | miR-5585 | SINE/Alu [41] | ||
| (366) | miR-5586 | LINE/L1 [41] | ||
| (367) | miR-5589 | DNA-TE/hAT-Tip100 [41] | ||
| (368) | miR-5590 | LINE/L1 [41] | ||
| (369) | miR-5591 | |||
| (370) | miR-562 | LINE/L1 [37] | ||
| (371) | miR-566 | SINE/Alu [37,41] | ||
| (372) | miR-568 | DNA-TE/Tc1-Mariner [37] | ||
| (373) | miR-5681a | SINE/MIR [41] | ||
| (374) | miR-5682 | LINE/L1 [41] | ||
| (375) | miR-5683 | DNA-TE/hAT-Charlie [41] | ||
| (376) | miR-5684 | SINE/Alu [41] | ||
| (377) | miR-5689 | |||
| (378) | miR-5691 | DNA-TE/hAT-Cahrlie [41] | ||
| (379) | miR-5694 | LTR/ERVL [41] | ||
| (380) | miR-5695 | LTR-ERV1 [41] | ||
| (381) | miR-5697 | LINE/L1 [41] | ||
| (382) | miR-5698 | |||
| (383) | miR-570 | DNA-TE/TcMar-Mariner [37,41,43] | ||
| (384) | miR-5700 | LINE/L2 [41] | ||
| (385) | miR-5706 | DNA/TcMar-Tigger [41] | ||
| (386) | miR-5708 | SINE/Alu [41] | ||
| (387) | miR-571 | LINE/L1 [37,41] | ||
| (388) | miR-575 | SINE [37] | ||
| (389) | miR-576 | LINE/L1 [29,37] | ↑: BLCA, BRCA, ESCA, HNSC, KICH, KIRC, KIRP, LUAD, LUSC, PRAD, READ, STAD, UCEC; ↓: CHOL, LIHC, THCA (tumor tissue) [44] | ↑ (blood plasma) [59] |
| (390) | miR-577 | LINE/L2 [33,37,41] | ↑: BLCA, CHOL, COAD, HNSC, KICH, LUAD, LUSC, READ, STAD, UCEC; ↓: KIRC, KIRP, THCA (tumor tissue) [44] | |
| (391) | miR-578 | LINE/CR1 [37] | ||
| (392) | miR-579 | DNA-TE/TcMar-Mariner [37,41] | ||
| (393) | miR-581 | DNA-TE/hAT-Charlie [33,37,41] | ||
| (394) | miR-582 | LINE/CR1 [29,35,37] | ↑: BRCA, COAD, KICH, PRAD, READ; ↓: CHOL, HNSC, LIHC, THCA (tumor tissue) [44] | |
| (395) | miR-584 | DNA-TE/hAT-Blackjack [29,35,37] | ↑: BLCA, ESCA, HNSC, KICH, KIRC, KIRP, PRAD, STAD; ↓: BRCA, LUAD, THCA (tumor tissue) [44] | |
| (396) | miR-585 | ERV-L/MaLR [37,41] | ↓: BRCA, KICH, KIRC, THCA (tumor tissue) [44] | ↑ (endothelial cells) [60] |
| (397) | miR-587 | DNA-TE/hAT [37] | ||
| (398) | miR-588 | LINE/L1 [37,41] | ||
| (399) | miR-591 | DNA-TE/hAT-Charlie [33,37,41] | ||
| (400) | miR-598 | DNA-TE/CACTA LP [37] | ||
| (401) | miR-603 | DNA-TE/TcMar-Mariner [37,41,43] | ||
| (402) | miR-606 | LINE/L1 [37,41] | ||
| (403) | miR-607 | SINE/MIR [29,37,41] | ||
| (404) | miR-608 | LINE/L2 [33,37] | ||
| (405) | miR-6088 | SINE/Alu [41] | ||
| (406) | miR-612 | SINE/MIR [33,37,41] | ||
| (407) | miR-6127 | SINE/MIR [41] | ||
| (408) | miR-6130 | LINE/L1 [41] | ||
| (409) | miR-616 | LINE/L2 [35,37,41] | ↑: KICH, KIRC, KIRP, LUSC, UCEC; ↓: CHOL, LIHC (tumor tissue) [44] | |
| (410) | miR-619 | LINE/L1; SINE/Alu [35,37,39,41,43] | ||
| (411) | miR-625 | LINE/L1 [35,37,41] | ||
| (412) | miR-626 | |||
| (413) | miR-630 | SINE/MIR [12] | ↓: IPF (lung tissue) [68] | |
| (414) | miR-6303 | DNA-TE/MADE1 [43] | ||
| (415) | miR-633 | SINE/MIR [35,37,41] | ||
| (416) | miR-634 | LINE/L1 [37,41] | ||
| (417) | miR-637 | LINE/L1 [33,37,41,43] | ||
| (418) | miR-638 | DNA-TE/hAT [37] | ||
| (419) | miR-640 | SINE/MIR [37,41] | ||
| (420) | miR-644a | LINE/L1 [37,41] | ||
| (421) | miR-645 | DNA-TE/hAT-Charlie [37,41] | ||
| (422) | miR-646 | LTR/ERVL [37,41] | ||
| (423) | miR-649 | DNA-TE/TcMar-Tigger [37,39,41] | ||
| (424) | miR-6500 | LTR/ERV1 [41] | ||
| (425) | miR-6503 | LTR/ERVL-MaLR [41] | ||
| (426) | miR-6507 | LINE/L1 [41] | ||
| (427) | miR-652 | DNA/hAT-Tip100 [29,35,37,43]. | ↑: BLCA, ESCA, HNSC, LIHC, STAD, THCA, UCEC; ↓: COAD, KICH, LUAD, LUSC, THCA (tumor tissue) [44] | |
| (428) | miR-659 | DNA-TE/hAT-Tip100 [37,41] | ||
| (429) | miR-663 | LINE/I [37] | ||
| (430) | miR-663b | LTR/Gypsy [37] | ||
| (431) | miR-6745 | LINE/L2 [41] | ||
| (432) | miR-6839 | LTR/ERV1 [41] | ||
| (433) | miR-6854 | DNA-TE/hAT-Charlie [41] | ||
| (434) | miR-6887 | LINE/L2 [41] | ||
| (435) | miR-708 | LINE/L2 [35,37,41] | ↑: BLCA, BRCA, CHOL, COAD, HNSC, KIRC, LUAD, LUSC, PRAD, READ, STAD; ↓: KICH, THCA (tumor tissue) [44]; IPF (lung tissue) [69] | ↑ (spleen tissue) [62] |
| (436) | miR-7151 | LINE/L1 [41] | ||
| (437) | miR-7157 | |||
| (438) | miR-720 | LTR/ERV1 [37] | ||
| (439) | miR-769 | LINE/CR1 [12] | ↑: BLCA, BRCA, ESCA, HNSC, KIRC, KIRP, LIHC, LUSC, PRAD, STAD, UCEC; ↓: COAD (tumor tissue) [44] | |
| (440) | miR-7702 | LINE/RTE-BovB [41] | ||
| (441) | miR-7849 | LTR/ERVL-MaLR [41] | ||
| (442) | miR-7850 | |||
| (443) | miR-7851 | SINE/Alu [41] | ||
| (444) | miR-7853 | DNA-TE/hAT-Cahrlie [41] | ||
| (445) | miR-7973-1 | SINE/MIR [41] | ||
| (446) | miR-7973-2 | |||
| (447) | miR-7975 | LTR/ERV1 [41] | ||
| (448) | miR-7977 | |||
| (449) | miR-7978 | LINE/L1 [41] | ||
| (450) | miR-8056 | LINE/L2 [41] | ||
| (451) | miR-8067 | LINE/L1 [41] | ||
| (452) | miR-8074 | |||
| (453) | miR-8076 | SINE/MIR [41] | ||
| (454) | miR-8079 | SINE/MIR [41] | ||
| (455) | miR-8084 | LINE/L1 [41] | ||
| (456) | miR-8086 | SINE/Alu [41] | ||
| (457) | miR-877 | LINE/L1 [37] | ||
| (458) | miR-885 | SINE/MIR [37,41] | ↑: KICH; ↓: CHOL (tumor tissue) [44] | ↑ (blood) [61] |
| (459) | miR-887 | LINE/L2 [37,41] | ↑: BRCA; ↓: HNSC, KICH, KIRP, PAAD, THCA (tumor tissue) [44] | |
| (460) | miR-891a | SINE/MIR [33,37,41] | ||
| (461) | miR-891b | |||
| (462) | miR-921 | SINE/MIR [37,41] | ||
| (463) | miR-924 | LTR/ERV1 [37] | ||
| (464) | miR-941-1 | LTR/Gypsy [37] | ||
| (465) | miR-941-3 | |||
| (466) | miR-941-4 | |||
| (467) | miR-95 | LINE/L2 [29,37,43] | ↑: CHOL, COAD, PRAD, READ, STAD, UCEC; ↓: HNSC, KICH, PCPG, THCA (tumor tissue) [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafin, R.N.; Khusnutdinova, E. Perspective for Studying the Relationship of miRNAs with Transposable Elements. Curr. Issues Mol. Biol. 2023, 45, 3122-3145. https://doi.org/10.3390/cimb45040204
Mustafin RN, Khusnutdinova E. Perspective for Studying the Relationship of miRNAs with Transposable Elements. Current Issues in Molecular Biology. 2023; 45(4):3122-3145. https://doi.org/10.3390/cimb45040204
Chicago/Turabian StyleMustafin, Rustam Nailevich, and Elza Khusnutdinova. 2023. "Perspective for Studying the Relationship of miRNAs with Transposable Elements" Current Issues in Molecular Biology 45, no. 4: 3122-3145. https://doi.org/10.3390/cimb45040204
APA StyleMustafin, R. N., & Khusnutdinova, E. (2023). Perspective for Studying the Relationship of miRNAs with Transposable Elements. Current Issues in Molecular Biology, 45(4), 3122-3145. https://doi.org/10.3390/cimb45040204






