The Use of Biomaterials in Three-Dimensional Culturing of Cancer Cells
Abstract
1. Introduction
2. Hydrogels
3. Natural Polymer Hydrogels
3.1. Gelatin Methacrylate (GelMa)
3.2. Agarose
3.3. Alginate
3.4. Collagen
4. Synthetic Polymer Hydrogel
4.1. Poly(N-Isopropyl Acrylamide) (PNIPAm)
4.2. Poly(Ethylene Glycol) (PEG)
4.3. Polyethylene Terephthalate (PET)
4.4. Poly-Lactic-co-Glicolyc Acid (PLGA)
5. Applications
5.1. Breast Cancer
5.1.1. Drug Sensitivity
5.1.2. Cell Morphology
5.1.3. Viability
5.2. Lung Cancer
5.2.1. Drug Sensitivity
5.2.2. Cell Morphology
5.2.3. Viability
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edmondson, R.; Broglie, J.; Adcock, A.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension-how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.; de Melo-Diogo, D.; Gaspar, V.; Carvalho, M.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Santini, M.T.; Rainaldi, G.; Romano, R.; Ferrante, A.; Clemente, S.; Motta, A.; Indovina, P.L. MG-63 human osteosarcoma cells grown in monolayer and as three-dimensional tumor spheroids present a different metabolic profile: A 1H NMR study. FEBS Lett. 2004, 557, 148–154. [Google Scholar] [CrossRef]
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental anti-tumor therapy in 3-D: Spheroids–Old hat or a new challenge? Int. J. Radiat. Biol. 2007, 83, 849–871. [Google Scholar] [CrossRef]
- Wästfelt, M.; Fadeel, B.; Henter, J.I. A journey of hope: Lessons learned from studies on rare diseases and orphan drugs. J. Intern. Med. 2006, 260, 1–10. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Gottardi, R.; De Riccardis, G.; Avolio, M.; Nichols, D.; Pirosa, A.; Alexander, P.; Raimondi, M.; Tuan, R. A 3D printed microfluidic bioreactor to engineer biphasic construct. In Food, Pharmaceutical and Bioengineering Division 2018—Core Programming, Proceedings of the Area 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 27–30 October 2018; AIChE: New York, NY, USA, 2018; Volume 2, pp. 980–981. [Google Scholar]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Giussani, M.; Merlino, G.; Cappelletti, V.; Tagliabue, E.; Daidone, M.G. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin. Cancer Biol. 2015, 35, 3–10. [Google Scholar] [CrossRef]
- Gomes, L.R.; Vessoni, A.; Menck, C.F.M. Three-dimensional microenvironment confer enhanced sensitivity to doxorubicin by reducing p53-dependent induction of autophagy. Oncogene 2015, 34, 5329–5340. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.; Freeman, J.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, T.-H. Recent Advances in Multicellular Tumor Spheroid Generation for Drug Screening. Biosensors 2021, 11, 445. [Google Scholar] [CrossRef]
- Prina-Mello, A.; Jain, N.; Liu, B.; Kilpatrick, J.I.; Tutty, M.A.; Bell, A.P.; Jarvis, S.P.; Volkov, Y.; Movia, D. Culturing substrates influence the morphological, mechanical and biochemical features of lung adenocarcinoma cells cultured in 2D or 3D. Tissue Cell 2018, 50, 15–30. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, X.; Liu, H.; Yang, L.; Yuan, X.; Chen, Y.; Wu, W.; Luo, J.; Long, J.; Huang, M.; et al. 3D printed porous microgel for lung cancer cells culture in vitro. Mater. Des. 2021, 210, 110079. [Google Scholar] [CrossRef]
- Quarta, A.; Gallo, N.; Vergara, D.; Salvatore, L.; Nobile, C.; Ragusa, A.; Gaballo, A. Investigation on the composition of agarose–collagen i blended hydrogels as matrices for the growth of spheroids from breast cancer cell lines. Pharmaceutics 2021, 13, 963. [Google Scholar] [CrossRef]
- Gomes, L.R.; Rocha, C.R.R.; Martins, D.J.; Fiore, A.P.Z.P.; Kinker, G.S.; Bruni-Cardoso, A.; Menck, C.F.M. ATR mediates cisplatin resistance in 3D-cultured breast cancer cells via translesion DNA synthesis modulation. Cell Death Dis. 2019, 10, 459. [Google Scholar] [CrossRef]
- Xin, X.; Yang, S.T. A Dual Fluorescent 3-D Multicellular Coculture of Breast Cancer MCF-7 and Fibroblast NIH-3T3 Cells for High Throughput Cancer Drug Screening. Biochem. Eng. J. 2019, 148, 152–161. [Google Scholar] [CrossRef]
- Gencoglu, M.F.; Barney, L.E.; Hall, C.L.; Brooks, E.A.; Schwartz, A.D.; Corbett, D.C.; Stevens, K.R.; Peyton, S.R. Comparative Study of Multicellular Tumor Spheroid Formation Methods and Implications for Drug Screening. ACS Biomater. Sci. Eng. vol. 2018, 4, 410–420. [Google Scholar] [CrossRef]
- Rijal, G.; Bathula, C.; Li, W. Application of Synthetic Polymeric Scaffolds in Breast Cancer 3D Tissue Cultures and Animal Tumor Models. Int. J. Biomater. 2017, 2017, 074890. [Google Scholar] [CrossRef] [PubMed]
- Shahriyari, F.; Janmaleki, M.; Sharifi, S.; Hesar, M.E.; Hoshian, S.; Taghiabadi, R.; Razaghian, A.; Ghadiri, M.; Peirovi, A.; Mahmoudi, M.; et al. Effect of cell imprinting on viability and drug susceptibility of breast cancer cells to doxorubicin. Acta Biomater. 2020, 113, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yazdimamaghani, M.; Vashaee, D.; Assefa, S.; Walker, K.J.; Madihally, S.V.; Köhler, G.A.; Tayebi, L. Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 911–931. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Roorda, W.E.; BoddÉ, H.; de Boer, A.; Junginger, H.E. Synthetic hydrogels as drug delivery systems. Pharm. Weekbl. 1986, 8, 165–189. [Google Scholar]
- Kopeček, J. Polymer chemistry: Swell gels. Nature 2002, 417, 388–391. [Google Scholar] [CrossRef]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef]
- Hajebi, S.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Hybrid and hollow Poly (N,N-dimethylaminoethyl methacrylate) nanogels as stimuli-responsive carriers for controlled release of doxorubicin. Polymer 2019, 180, 121716. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Brandl, F.; Sommer, F.; Goepferich, A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials 2007, 28, 134–146. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Cruise, G.M.; Scharp, D.; Hubbell, J.A. Characterization of permeability and network structure of interfacially photopolymerized poly (ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Schacht, E.; Dubruel, P. Reversible gelatin-based hydrogels: Finetuning of material properties. Eur. Polym. J. 2011, 47, 1039–1047. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. Biomater. Appl. Nanomed. 2011, 2, 17–52. [Google Scholar]
- Balgude, A.P.; Yu, X.; Szymanski, A.; Bellamkonda, R.V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 2001, 22, 1077–1084. [Google Scholar] [CrossRef]
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic encapsulation of cells in polymer microgels. Small 2012, 8, 1633–1642. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.; Amici, E.; Plucknett, K.; Aymard, P. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef]
- Xu, X.; Farach-Carson, M.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Ji, W.; Chen, X.; Li, C.; Ge, R. Enrichment of cancer stem cells by agarose multi-well dishes and 3D spheroid culture. Cell Tissue Res. 2019, 375, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Stokols, S.; Sakamoto, J.; Breckon, C.; Holt, T.; Weiss, J.; Tuszynski, M.H. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006, 12, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.M.; Ng, S.F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Erratum: Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Seagle, C.; Rice, L.; Macdonald, J.; Gerber, D.A. Functional analysis of encapsulated hepatic progenitor cells. Tissue Eng. 2006, 12, 2001–2008. [Google Scholar] [CrossRef]
- Read, G.H.; Miura, N.; Carter, J.L.; Kines, K.T.; Yamamoto, K.; Devasahayam, N.; Cheng, J.Y.; Camphausen, K.A.; Krishna, M.C.; Kesarwala, A.H. Three-dimensional alginate hydrogels for radiobiological and metabolic studies of cancer cells. Colloids Surf. B Biointerfaces 2018, 171, 197–204. [Google Scholar] [CrossRef]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel Blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Friess, W. Collagen–Biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Kuo, C.K.; Tuan, R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1615–1627. [Google Scholar] [CrossRef]
- Bian, W.; Liau, B.; Badie, N.; Bursac, N. Mesoscopic hydrogel molding to control the 3d geometry of bioartificial muscle tissues. Nat. Protoc. 2009, 4, 1522–1534. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Graziano, G. On the temperature-induced coil to globule transition of poly-N-isopropylacrylamide in dilute aqueous solutions. Int. J. Biol. Macromol. 2000, 27, 89–97. [Google Scholar] [CrossRef]
- Yadavalli, T.; Ramasamy, S.; Chandrasekaran, G.; Michael, I.; Therese, H.; Chennakesavulu, R. Dual responsive PNIPAM-chitosan targeted magnetic nanopolymers for targeted drug delivery. J. Magn. Magn. Mater. 2015, 380, 315–320. [Google Scholar] [CrossRef]
- Morris, C.; Szczupak, B.; Klymchenko, A.; Ryder, A.G. Study of water adsorption in poly(N-isopropylacrylamide) thin films using fluorescence emission of 3-hydroxyflavone probes. Macromolecules 2010, 43, 9488–9494. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly (N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Oh, Y.; Cha, J.; Kang, S.; Kim, P. A polyethylene glycol-based hydrogel as macroporous scaffold for tumorsphere formation of glioblastoma multiforme. J. Ind. Eng. Chem. 2016, 39, 10–15. [Google Scholar] [CrossRef]
- Lin, L.; Marchant, R.; Zhu, J.; Kottke-Marchant, K. Extracellular matrix-mimetic poly(ethylene glycol) hydrogels engineered to regulate smooth muscle cell proliferation in 3-D. Acta Biomater. 2014, 10, 5106–5115. [Google Scholar] [CrossRef] [PubMed]
- Keys, K.B.; Andreopoulos, F.; Peppas, N.A. Poly (ethylene glycol) star polymer hydrogels. Macromolecules 1998, 31, 8149–8156. [Google Scholar] [CrossRef]
- Khang, A.; Rodriguez, A.G.; Schroeder, M.E.; Sansom, J.; Lejeune, E.; Anseth, K.S.; Sacks, M.S. Quantifying heart valve interstitial cell contractile state using highly tunable poly (ethylene glycol) hydrogels. Acta Biomater. 2019, 96, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Benmeridja, L.; Giol, E.D.; Van der Meeren, L.; Van Damme, L.; Liu, Z.; Toncheva, A.; Raquez, J.-M.; Van den Brande, N.; Skirtach, A.; et al. Potential of poly (alkylene terephthalate)s to control endothelial cell adhesion and viability. Mater. Sci. Eng. C 2021, 129, 112378. [Google Scholar] [CrossRef]
- Subramaniam, A.; Sethuraman, S. Biomedical Applications of Nondegradable Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gao, R.; Pan, H.; Lian, J. Recent advances in the discovery, characterization, and engineering of poly (ethylene terephthalate) (PET) hydrolases. Enzym. Microb. Technol. 2021, 150, 109868. [Google Scholar] [CrossRef]
- Ramachandran, B.; Chakraborty, S.; Dixit, M.; Muthuvijayan, V. A comparative study of polyethylene terephthalate surface carboxylation techniques: Characterization, in vitro haemocompatibility and endothelialization. React. Funct. Polym. 2018, 122, 22–32. [Google Scholar] [CrossRef]
- Osuchowska, P.N.; Ostrowski, R.; Sarzyński, A.; Strzelec, M.; Mierczyk, Z.; Trafny, E.A. Microstructured polyethylene terephthalate (PET) for microsieving of cancer cells. Results Phys. 2019, 15, 102612. [Google Scholar] [CrossRef]
- Caykara, T.; Silva, J.; Fernandes, S.; Braga, A.; Rodrigues, J.; Rodrigues, L.R.; Silva, C. Modification of PET surfaces with gum Arabic towards its bacterial anti-adhesiveness using an experimental factorial design approach. Mater. Today Commun. 2021, 28, 102684. [Google Scholar] [CrossRef]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Saremy, S.; Dorkoosh, F.A. Co-delivery of 5-fluorouracil and oxaliplatin in novel poly (3-hydroxybutyrate-co-3-hydroxy valerate acid)/poly (lactic-co-glycolic acid) nanoparticles for colon cancer therapy. Int. J. Biol. Macromol. 2019, 124, 1299–1311. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, T.; Yuan, Y.; Kłodzińska, S.N.; Zheng, T.; Sternberg, C.; Nielson, H.M.; Sun, Y.; Wan, F. Synthesis of carbon quantum dot-poly lactic-co-glycolic acid hybrid nanoparticles for chemo-photothermal therapy against bacterial biofilms. J. Colloid Interface Sci. 2020, 577, 66–74. [Google Scholar] [CrossRef]
- Yildiz-Ozturk, E.; Gulce-Iz, S.; Anil, M.; Yesil-Celiktas, O. Cytotoxic responses of carnosic acid and doxorubicin on breast cancer cells in butterfly-shaped microchips in comparison to 2D and 3D culture. Cytotechnology 2017, 69, 337–347. [Google Scholar] [CrossRef]
- Dhamecha, D.; Le, D.; Chakravarty, T.; Perera, K.; Dutta, A.; Menon, J.U. Fabrication of PNIPAm-based thermoresponsive hydrogel microwell arrays for tumor spheroid formation. Mater. Sci. Eng. C 2021, 125, 112100. [Google Scholar] [CrossRef]
- Deepa, P.R.; Vandhana, S.; Jayanthi, U.; Krishnakumar, S. Therapeutic and Toxicologic Evaluation of Anti-Lipogenic Agents in Cancer Cells Compared with Non-Neoplastic Cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 494–503. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic moduli of normal and pathological human breast tissues: An inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 2007, 52, 1565–1576. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.T. High-throughput 3-D cell-based proliferation and cytotoxicity assays for drug screening and bioprocess development. J. Biotechnol. 2011, 151, 186–193. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Monteiro, M.V.; Gaspar, V.; Ferreira, L.; Mano, J.F. Hydrogel 3D: In vitro tumor models for screening cell aggregation mediated drug response. Biomater. Sci. 2020, 8, 1855–1864. [Google Scholar] [CrossRef]
- Anton, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
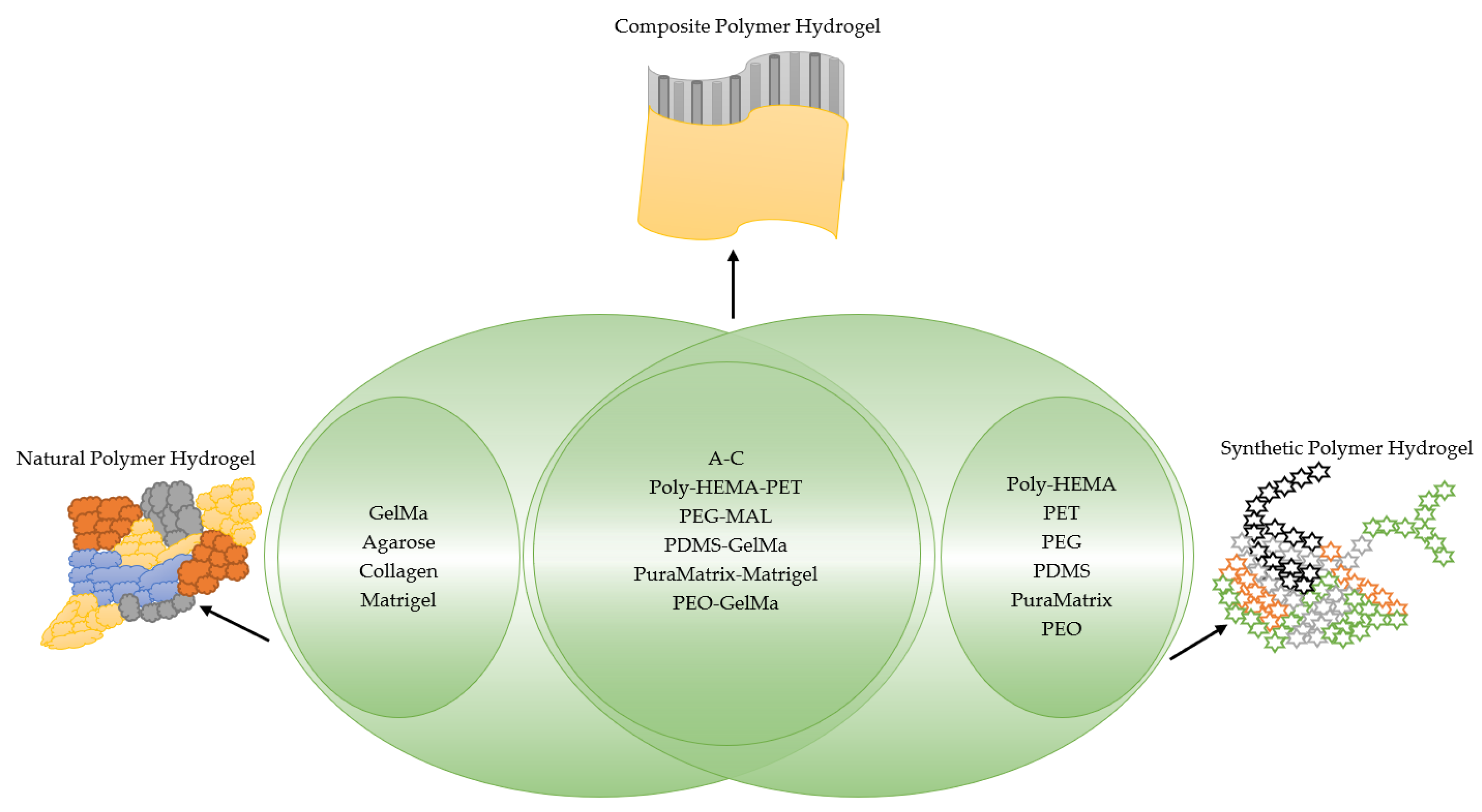
| Biomaterials | Drug Screening | Cell Line | Cancer Cells |
|---|---|---|---|
| Agarose–collagen (A–C) | Cisplatin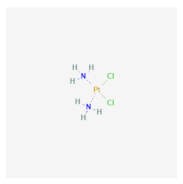 | MCF-7, MDA-MB-361, and MDA-MB-231 [18] | Breast cancer |
| Poly(2-hydroxyethyl methacrylate) (poly-HEMA) | Cisplatin | MCF-7 [19] | Breast cancer |
| Polyethylene terephthalate (PET) | Cisplatin Tamoxifen 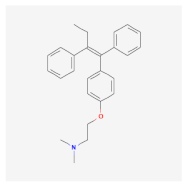 Oxaliplatin 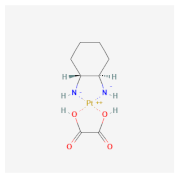 | MCF-7 and mouse fibroblast NIH-3T3 [20] | Breast cancer |
| PEG-Maleimide (PEG-MAL) | Cisplatin Paclitaxel 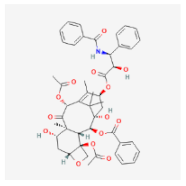 Sorafenib  Mafosfamide 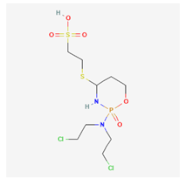 | MCF-7, AU565, BT549, LNCaPcol, PC-3, SKOV-3, OVCAR-3, HCC 1419, HCC 1428, MDA-MB 231, SkBr3, ZR-75-1, BT474 [21] | Breast cancer, Prostate cancer, Ovarian cancer |
| Polydimethylsiloxane (PDMS) and gelatin methacryloyl (GelMA) hydrogel | doxorubicin (DOX) | MCF7, SKBR3, dan ZR-75-1 [23] | Breast cancer |
| Polydimethylsiloxane (PDMS) | doxorubicin (DOX) | MCF-7 dan MDA-MB 231 [74] | Breast cancer |
| Poly-Lactic-co-Glicolyc Acid (PLGA) | 4-hydroxytamoxifen (4-HT)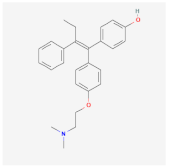 | T47D [22] | Breast cancer |
| poly N-isopropyl acrylamide-based hydrogel microwell array (PHMA) | Doxorubicin (DOX) | A549, MG-63 Hela and Human Lung Fibroblast (HLF) [75] | Lung cancer |
| PuraMatrix™ and Matrigel™ | Decotaxel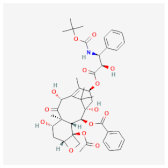 | A549 [16] | Lung Cancer |
| Polyethylene oxide—Gelatin methacryloyl (PEO-GelMA) | Paclitaxel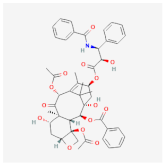 | LL/2 cells (mouse Lewis lung carcinoma), A549 cells (human lung carcinoma) and NCI-H1975 cells (human lung adenocarcinoma) [17] | Lung Cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanasti, N.; Faridah, L.; Fibriani, A.; Wiraswati, H.L.; Kusumawaty, D.; Ekawardhani, S. The Use of Biomaterials in Three-Dimensional Culturing of Cancer Cells. Curr. Issues Mol. Biol. 2023, 45, 1100-1112. https://doi.org/10.3390/cimb45020073
Hanasti N, Faridah L, Fibriani A, Wiraswati HL, Kusumawaty D, Ekawardhani S. The Use of Biomaterials in Three-Dimensional Culturing of Cancer Cells. Current Issues in Molecular Biology. 2023; 45(2):1100-1112. https://doi.org/10.3390/cimb45020073
Chicago/Turabian StyleHanasti, Novia, Lia Faridah, Azzania Fibriani, Hesti Lina Wiraswati, Diah Kusumawaty, and Savira Ekawardhani. 2023. "The Use of Biomaterials in Three-Dimensional Culturing of Cancer Cells" Current Issues in Molecular Biology 45, no. 2: 1100-1112. https://doi.org/10.3390/cimb45020073
APA StyleHanasti, N., Faridah, L., Fibriani, A., Wiraswati, H. L., Kusumawaty, D., & Ekawardhani, S. (2023). The Use of Biomaterials in Three-Dimensional Culturing of Cancer Cells. Current Issues in Molecular Biology, 45(2), 1100-1112. https://doi.org/10.3390/cimb45020073






