Possible Mechanisms of the Neuroprotective Actions of Date Palm Fruits Aqueous Extracts against Valproic Acid-Induced Autism in Rats
Abstract
1. Background
2. Materials and Methods
2.1. Preparation of Date Palm Fruit Aqueous Extract
2.2. Experimental Protocol
- Group I. Naïve control: the offspring male rats in this group received no treatment.
- Group II. Naïve control + AFE: the mothers (from embryonic E12.5th day until labor) and offspring male rats in this group were treated with aqueous fruit extracts (AFE) (4 mg/kg dissolved in 1 mL saline/day via gastric gavage) until PND45 [28].
- Group III. VPA group: the pregnant female rats in this group received single intraperitoneal dose of valproic acid (VPA) (500 mg/Kg) at E12.5th embryonic day
- Group IV. VPA + AFE: the offspring male rats in these groups were treated with VPA (500 mg/kg single i.p. injection at day 12.5) and aqueous extracts of palm date fruits to the mother (4 mg/kg dissolved in 1 mL saline/day via gastric gavage) until the time of labor and to the offspring until PND45 [28].
2.3. Behavioral Tests
Rotarod Test
2.4. Elevated Plus-Maze Test
2.5. Morris Water Maze Test
2.6. Harvesting of Brain Tissues
2.7. Assay of Oxidative Stress Markers (MDA, Catalase Activity, and Total Antioxidants) in Brain Tissues
2.8. Assessment of the Expression of the Antioxidant Transcription Factor (Nrf2) and HO-1 at the Level of mRNA by Real-Time PCR
2.9. Hematoxylin and Eosin Staining
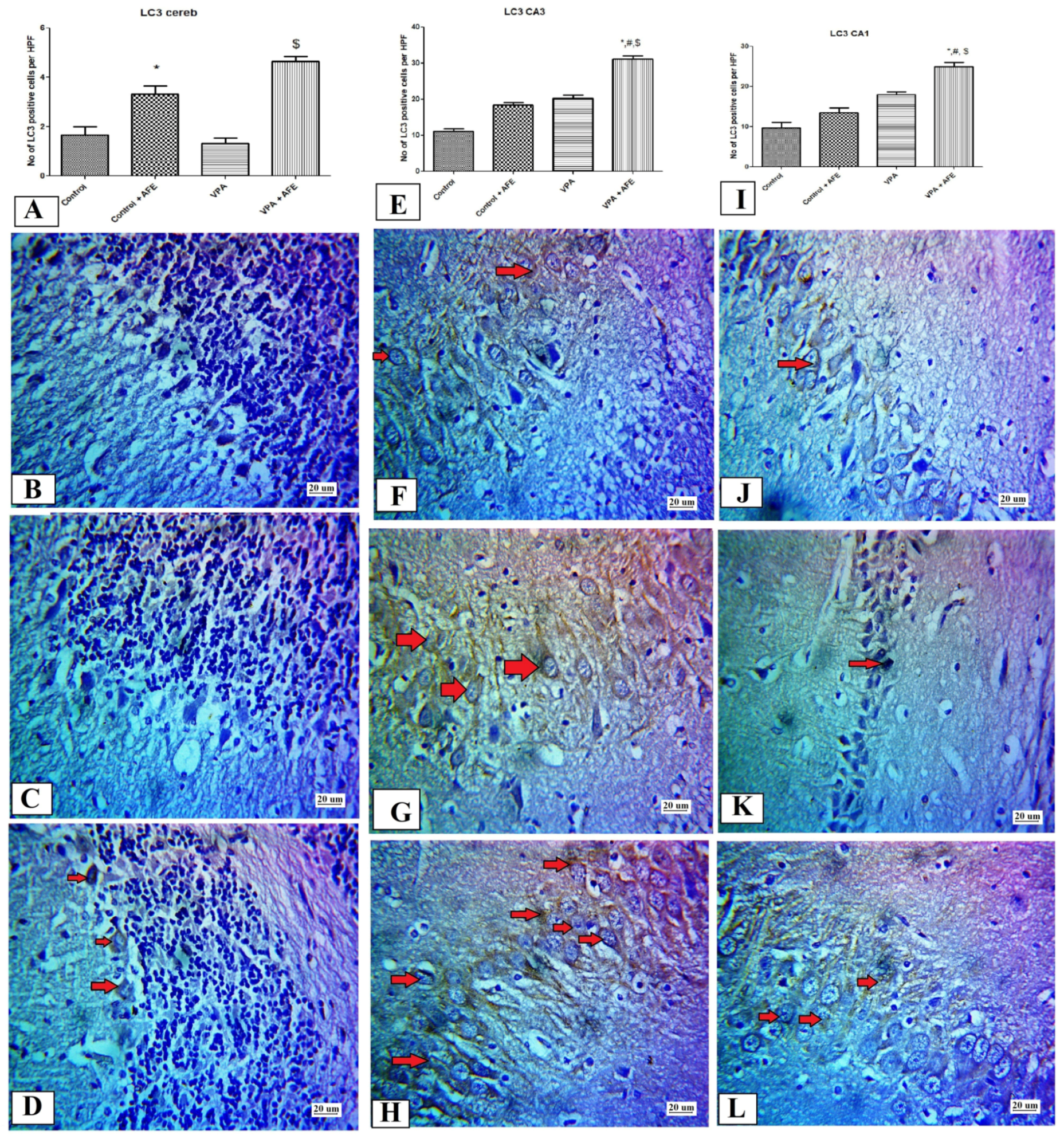
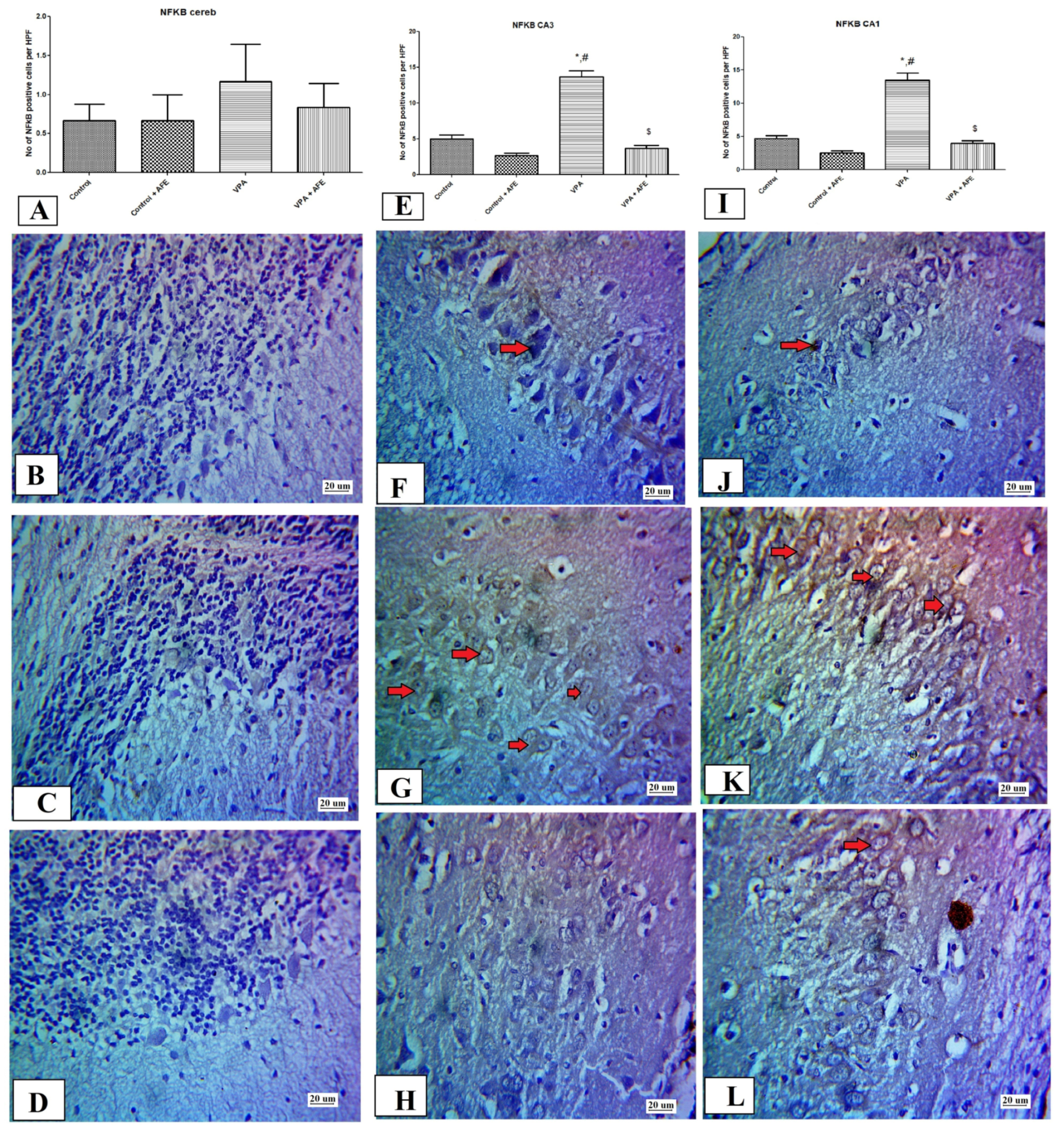
2.10. Immunohistochemical Examination for NF-κB, Caspase 3, and Sirt-1, and LC3 in CA3 and CA1 Regions of the Hippocampus and Cerebellum
2.11. Statistical Analysis
3. Results
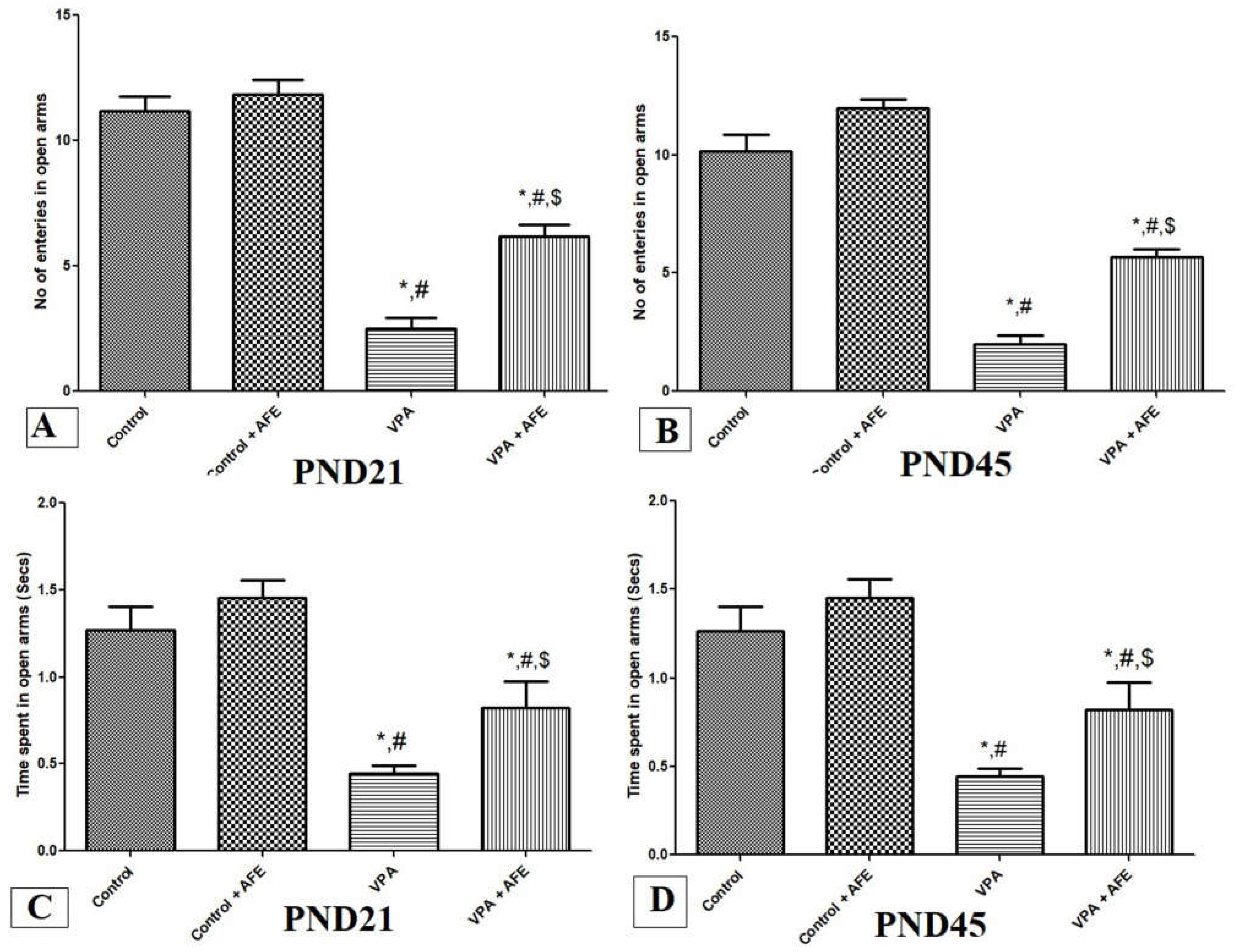
3.1. Effects of AFE on Spatial Memory Deficits and Motor Coordination in VPA-Induced Autism
3.2. Effects of AFE on Anxiety in VPA-Induced Autism
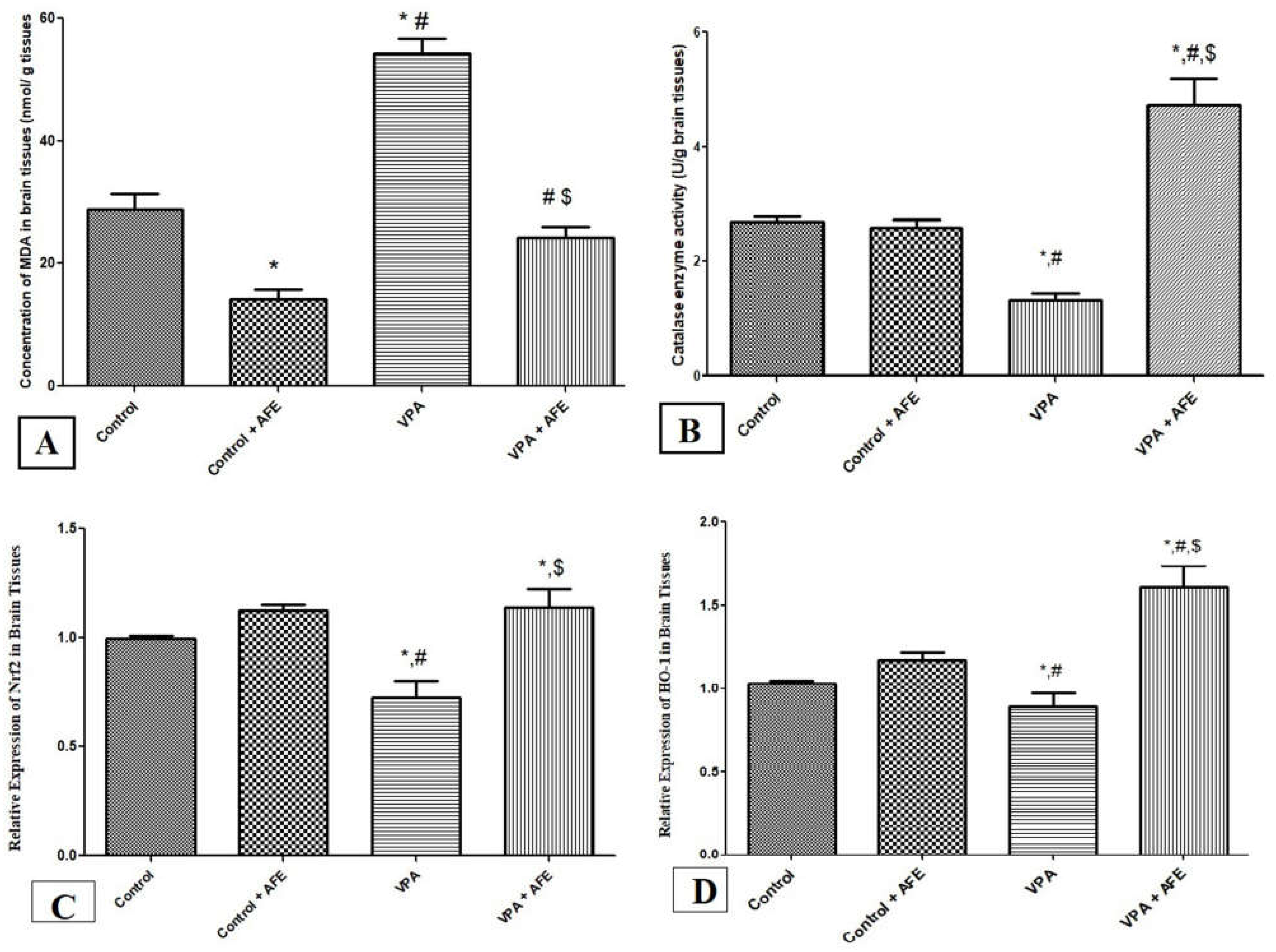
3.3. Effects of AFE on Oxidative Stress Markers (MDA and Catalase) and Expression of Nrf2 and HO-1 in VPA-Induced Autism
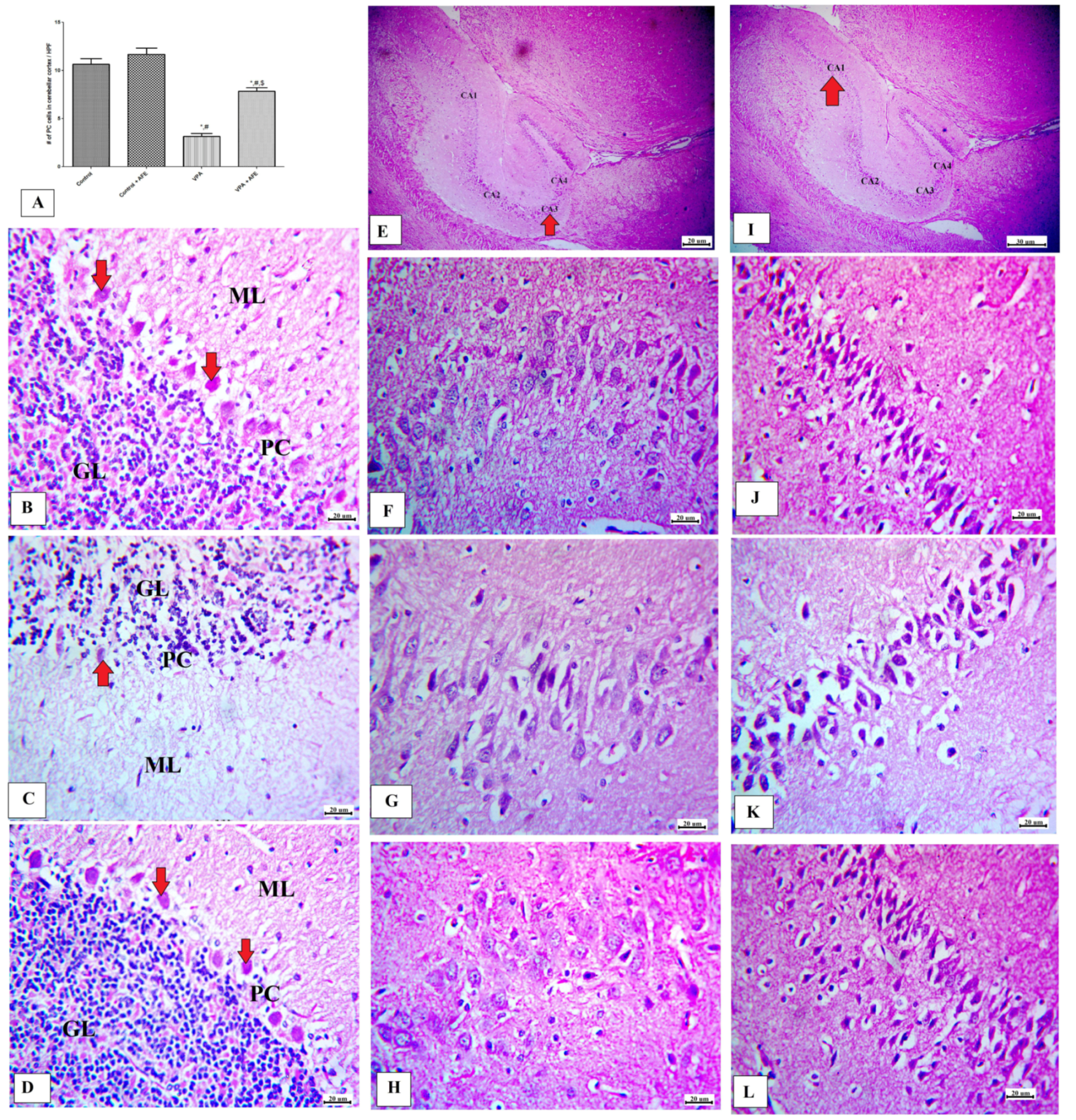
3.4. Effects of AFE on Cerebellar and Hippocampus Morphology in VPA-Induced Autism
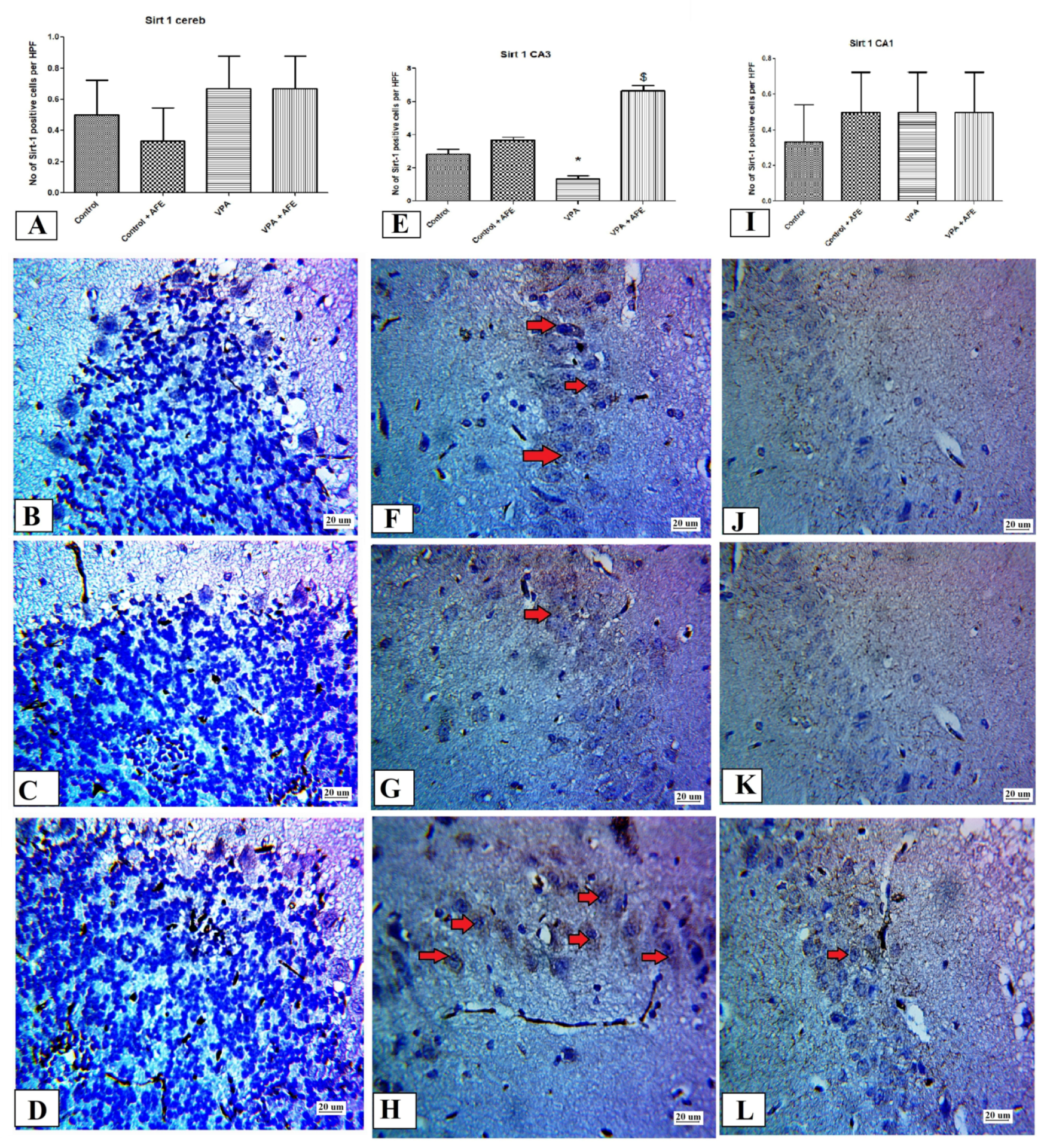
3.5. Effects of AFE on the Expression of Apoptotic Marker (Caspase-3), Sirt-1, Inflammatory Marker (NFκB), and Autophagic Marker (LC3) by Immunohistochemistry in Cerebellum and Hippocampus in VPA-Induced Autism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, H.; Oreve, M.-J.; Speranza, M. Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Arch. Pédiatr. 2018, 25, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Asadi-Shekaari, M.; Basiri, M.; Parvan, M.; Shabani, M.; Nozari, M. Improvement of autistic-like behaviors in adult rats prenatally exposed to valproic acid through early suppression of NMDA receptor function. Psychopharmacology 2019, 237, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chauhan, A.; Sahu, J.; Jaiswal, N.; Kumar, K.; Agarwal, A.; Kaur, J.; Singh, S. Prevalence of autism spectrum disorder in Indian children: A systematic review and meta-analysis. Neurol. India 2019, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, M.; Stockton, M.; Zhao, X. Hippocampal deficits in neurodevelopmental disorders. Neurobiol. Learn. Mem. 2018, 165, 106945. [Google Scholar] [CrossRef]
- Dubovický, M. Neurobehavioral manifestations of developmental impairment of the brain. Interdiscip. Toxicol. 2010, 3, 59–67. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Partner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Alsdorf, R.; Wyszynski, D.F. Teratogenicity of sodium valproate. Expert Opin. Drug Saf. 2005, 4, 345–353. [Google Scholar] [CrossRef]
- Arndt, T.L.; Stodgell, C.J.; Rodier, P.M. The teratology of autism. Int. J. Dev. Neurosci. 2005, 23, 189–199. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef]
- Rodier, P.M. Converging evidence for brain stem injury in autism. Dev. Psychopathol. 2002, 14, 537–557. [Google Scholar] [CrossRef]
- Kemper, T.L.; Bauman, M. Neuropathology of Infantile Autism. J. Neuropathol. Exp. Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Ingram, J.L.; Peckham, S.M.; Tisdale, B.; Rodier, P.M. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol. Teratol. 2000, 22, 319–324. [Google Scholar] [CrossRef]
- vanSteensel, F.J.; Bogels, S.M.; Perrin, S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clin. Child Fam. Psychol. Rev. 2011, 14, 302–317. [Google Scholar] [CrossRef]
- Sui, L.; Chen, M. Prenatal exposure to valproic acid enhances synaptic plasticity in the medial prefrontal cortex and fear memories. Brain Res. Bull. 2012, 87, 556–563. [Google Scholar] [CrossRef]
- Settipani, C.A.; Puleo, C.M.; Conner, B.; Kendall, P.C. Characteristics and anxiety symptom presentation associated with autism spectrum traits in youth with anxiety disorders. J. Anxiety Disord. 2012, 26, 459–467. [Google Scholar] [CrossRef]
- Kataoka, S.; Takuma, K.; Hara, Y.; Maeda, Y.; Ago, Y.; Matsuda, T. Autism-like behaviours with transient histone hyper-acetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Sandhya, T.; Sowjanya, J.; Veeresh, B. Bacopa monniera (L.) Wettst Ameliorates Behavioral Alterations and Oxidative Markers in Sodium Valproate Induced Autism in Rats. Neurochem. Res. 2012, 37, 1121–1131. [Google Scholar] [CrossRef]
- Gottfried, C.; Quincozes-Santos, A.; Basli, K.; Richard, T. Resveratrol and Neuroprotection. In Resveratrol: Sources, Production and Health Benefits; Nova Publisher: New York, NY, USA, 2012. [Google Scholar]
- Lu, M.-J.; Chen, C. Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Res. Int. 2008, 41, 130–137. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef]
- Pragnya, B.; Kameshwari, J.S.L.; Veeresh, B. Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/C mice. Behav. Brain Res. 2014, 270, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Aktoz, T.; Kanter, M.; Aktas, C. Protective effects of quercetin on testicular torsion/detorsion-induced ischaemia-reperfusion injury in rats. Andrologia 2010, 42, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Vayalil, P.K. Date Fruits (Phoenix Dactylifera Linn): An Emerging Medicinal Food. Crit. Rev. Food Sci. Nutr. 2012, 52, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Zain, M.R.A.M.; Kari, Z.A.; Dawood, M.A.O.; Ariff, N.S.N.A.; Salmuna, Z.N.; Ismail, N.; Ibrahim, A.H.; Krishnan, K.T.; Mat, N.F.C.; Edinur, H.A.; et al. Bioactivity and Pharmacological Potential of Date Palm (Phoenix dactylifera L.) Against Pandemic COVID-19: A Comprehensive Review. Appl. Biochem. Biotechnol. 2022, 194, 4587–4624. [Google Scholar] [CrossRef]
- El-Mousalamy, A.M.; Hussein, A.A.M.; Mahmoud, S.A.; Abdelaziz, A.; Shaker, G. Aqueous and methanolic extracts of palm date seeds and fruits (Phoenix dactylifera) protects against diabetic nephropathy in type II diabetic rats. Biochem. Physiol. Open Access 2016, 5, 205. [Google Scholar]
- Alghamdi, M.A.; Hussein, A.M.; Al-Eitan, L.N.; Elnashar, E.; Elgendy, A.; Abdalla, A.M.; Ahmed, S.; Khalil, W.A. Possible mechanisms for the renoprotective effects of date palm fruits and seeds extracts against renal ischemia/reperfusion injury in rats. Biomed. Pharmacother. 2020, 130, 110540. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2004, 30, 80–89. [Google Scholar] [CrossRef]
- Barendse, E.M.; Hendriks, M.P.; Jansen, J.F.; Backes, W.H.; Hofman, P.A.; Thoonen, G.; Aldenkamp, A.P. Working memory deficits in high-functioning adolescents with autism spectrum disorders: Neuropsychological and neuroimaging correlates. J. Neurodev. Disord. 2013, 5, 14. [Google Scholar] [CrossRef]
- Morakotsriwan, N.; Wattanathorn, J.; Kirisattayakul, W.; Chaisiwamongkol, K. Autistic-Like Behaviors, Oxidative Stress Status, and Histopathological Changes in Cerebellum of Valproic Acid Rat Model of Autism Are Improved by the Combined Extract of Purple Rice and Silkworm Pupae. Oxid. Med. Cell. Longev. 2016, 2016, 3206561. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Hussein, A.M.; Adel, M.; El-Mesery, M.; Abbas, K.M.; Ali, A.N.; Abulseoud, O.A. l-Carnitine Modulates Epileptic Seizures in Pentylenetetrazole-Kindled Rats via Suppression of Apoptosis and Autophagy and Upregulation of Hsp70. Brain Sci. 2018, 8, 45. [Google Scholar] [CrossRef]
- Al-Gholam, A.M.; Ameen, O. The Neuroprotective Effect of Ginkgo Biloba Extract on Valproic Acid Induced Autistic Features in Mice. J. Clin. Diagn. Res. 2020, 14, KF01–KF06. [Google Scholar] [CrossRef]
- Rapoport, M.; van Reekum, R.; Mayberg, H. The role of the cerebellum in cognition and behavior: A selective review. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 193–198. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int. Rev. Psychiatry 2001, 13, 247–260. [Google Scholar] [CrossRef]
- Singh, V.K. Neuro-immunopathogenesis in autism. NeuroImmune Biol. 2001, 1, 447–458. [Google Scholar] [CrossRef]
- Schneider, T.; Ziòłkowska, B.; Gieryk, A.; Tyminska, A.; Przewłocki, R. Prenatal exposure to valproic acid disturbs the enkephalinergic system functioning, basal hedonic tone, and emotional responses in an animal model of autism. Psychopharmacology 2007, 193, 547–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Wang, F.; Wang, Z.; Peng, Y.; Li, R. Down regulating the canonical wnt/β-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochem. Res. 2012, 37, 1409–1419. [Google Scholar] [CrossRef]
- Essa, M.M.; Braidy, N.; Bridge, W.; Subash, S.; Manivasagam, T.; Vijayan, R.; Al-Adawi, S.; Guillemin, G. Review of natural products on Parkinson’s disease pathology. J. Aging Res. Clin. Pract. 2014, 3, 127–136. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neuro-degenerative diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar]
- Li, M.; Chen, L.; Lee, H.S.; Yu, L.; Zhang, Y. The role of intracellular amyloid beta in Alzheimer’s disease. Prog. Neurobiol. 2007, 83, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.S.; Marzieh, P.; Shahriar, D.; Zahed, S.K.; Pari, K.T. Neuroprotective effects of aqueous date fruit extract on focal cerebral ischemia in rats. Pak. J. Med. Sci. 2008, 24, 661–665. [Google Scholar]
- Pujari, R.R.; Vyawahare, N.S.; Thakurdesai, P.A. Neuroprotective and antioxidant role of Phoenix dactylifera in permanent bilateral common carotid occlusion in rats. J. Acute Dis. 2014, 3, 104–114. [Google Scholar] [CrossRef]
- Tung, E.W.Y.; Winn, L.M. Valproic Acid Increases Formation of Reactive Oxygen Species and Induces Apoptosis in Postimplantation Embryos: A Role for Oxidative Stress in Valproic Acid-Induced Neural Tube Defects. Mol. Pharmacol. 2011, 80, 979–987. [Google Scholar] [CrossRef]
- Baldaçara, L.; Borgio, J.G.F.; de Lacerda, A.L.T.; Jackowski, A.P. Cerebellum and psychiatric disorders. Rev. Bras. Psiquiatr. 2008, 30, 281–289. [Google Scholar] [CrossRef]
- Taleb, A.; Lin, W.; Xu, X.; Zhang, G.; Zhou, Q.G.; Naveed, M.; Han, F. Emerging mechanisms of valproic ac-id-induced neurotoxic events in autism and its implications for pharmacological treatment. Biomed. Pharmacother. 2021, 137, 111322. [Google Scholar] [CrossRef]
- Edobor, H.D.; Musa, S.A.; Umana, U.E.; Oderinde, G.P.; Agbon, A.N. Neuroprotective effect of phoenix dactylifera (date palm) on paraquat triggered cortico-nigral neurotoxicity. J. Neurobehav. Sci. 2021, 8, 199–208. [Google Scholar]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014, 11, 189. [Google Scholar] [CrossRef]
- Gottfried, C.; Bambini, V., Jr.; Francis, F.; Riesgo, R.; Savino, W. The Impact of Neuroimmune Alterations in Autism Spectrum Disorder. Front. Psychiatry 2015, 6, 121. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Wang, H.; Yin, Y.-X.; Gong, D.-M.; Hong, L.-J.; Wu, G.; Jiang, Q.; Wang, C.-K.; Blinder, P.; Long, S.; Han, F.; et al. Cathepsin B inhibition ameliorates leukocyte-endothelial adhesion in the BTBR mouse model of autism. CNS Neurosci. Ther. 2018, 25, 476–485. [Google Scholar] [CrossRef]
- Essa, M.M.; Subash, S.; Dhanalakshmi, C.; Manivasagam, T.; Al-Adawi, S.; Guillemin, G.J.; Thenmozhi, A.J. Dietary supplementation of walnut partially reverses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced neurodegeneration in a mouse model of Parkinson’s disease. Neurochem. Res. 2015, 40, 1283–1293. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Lai, Y.-l.; Li, X.-t.; Fu, Y.-C.; Xu, W.-C. SIRT1 interacts with metabolic transcriptional factors in the pancreas of insulin-resistant and calorie-restricted rats. Mol. Biol. Reports. 2013, 40, 3373–3380. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2013, 25, 138–145. [Google Scholar] [CrossRef]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef]
- El Nashar, E.M.; Obydah, W.; Alghamdi, M.A.; Saad, S.; Yehia, A.; Maryoud, A.; Kiwan, N.A.; Alasmari, W.A.; Hussein, A.M. Effects of Stevia rebaudiana Bertoni extracts in the rat model of epilepsy induced by pentylenetetrazol: Sirt-1, at the crossroads between inflammation and apoptosis. J. Integr. Neurosci. 2022, 21, 21. [Google Scholar] [CrossRef]
- Kaewmool, C.; Kongtawelert, P.; Phitak, T.; Pothacharoen, P.; Udomruk, S. Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-κB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 2020, 341, 577164. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Wu, Q.; Wu, L.-Y.; Ye, Z.-N.; Jiang, T.-W.; Li, W.; Zhuang, Z.; Zhou, M.-L.; Zhang, X.; Hang, C.-H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, A.M.; Mahmoud, S.A.; Elazab, K.M.; Abouelnaga, A.F.; Abass, M.; Mosa, A.A.H.; Hussein, M.A.M.; Elsayed, M.E.G. Possible Mechanisms of the Neuroprotective Actions of Date Palm Fruits Aqueous Extracts against Valproic Acid-Induced Autism in Rats. Curr. Issues Mol. Biol. 2023, 45, 1627-1643. https://doi.org/10.3390/cimb45020105
Hussein AM, Mahmoud SA, Elazab KM, Abouelnaga AF, Abass M, Mosa AAH, Hussein MAM, Elsayed MEG. Possible Mechanisms of the Neuroprotective Actions of Date Palm Fruits Aqueous Extracts against Valproic Acid-Induced Autism in Rats. Current Issues in Molecular Biology. 2023; 45(2):1627-1643. https://doi.org/10.3390/cimb45020105
Chicago/Turabian StyleHussein, Abdelaziz M., Seham Ahmed Mahmoud, Khalid Mohammed Elazab, Ahmed F. Abouelnaga, Marwa Abass, Ahmed A. H. Mosa, Mennatullah A. M. Hussein, and Mohamed E. G. Elsayed. 2023. "Possible Mechanisms of the Neuroprotective Actions of Date Palm Fruits Aqueous Extracts against Valproic Acid-Induced Autism in Rats" Current Issues in Molecular Biology 45, no. 2: 1627-1643. https://doi.org/10.3390/cimb45020105
APA StyleHussein, A. M., Mahmoud, S. A., Elazab, K. M., Abouelnaga, A. F., Abass, M., Mosa, A. A. H., Hussein, M. A. M., & Elsayed, M. E. G. (2023). Possible Mechanisms of the Neuroprotective Actions of Date Palm Fruits Aqueous Extracts against Valproic Acid-Induced Autism in Rats. Current Issues in Molecular Biology, 45(2), 1627-1643. https://doi.org/10.3390/cimb45020105





