Liver Damage and microRNAs: An Update
Abstract
1. Introduction
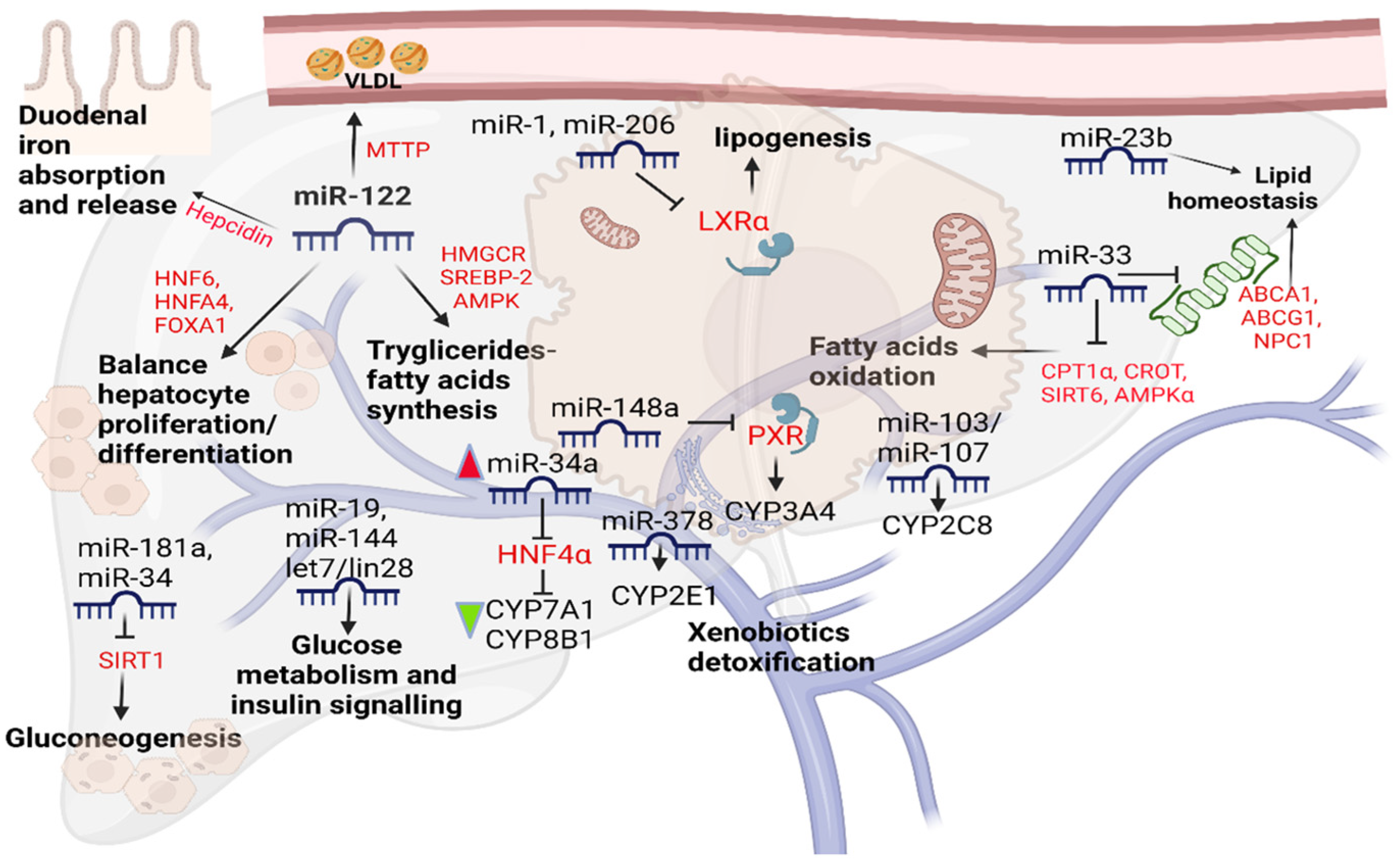
2. Endogenous Liver microRNAs
3. Drug-Induced Liver Damage
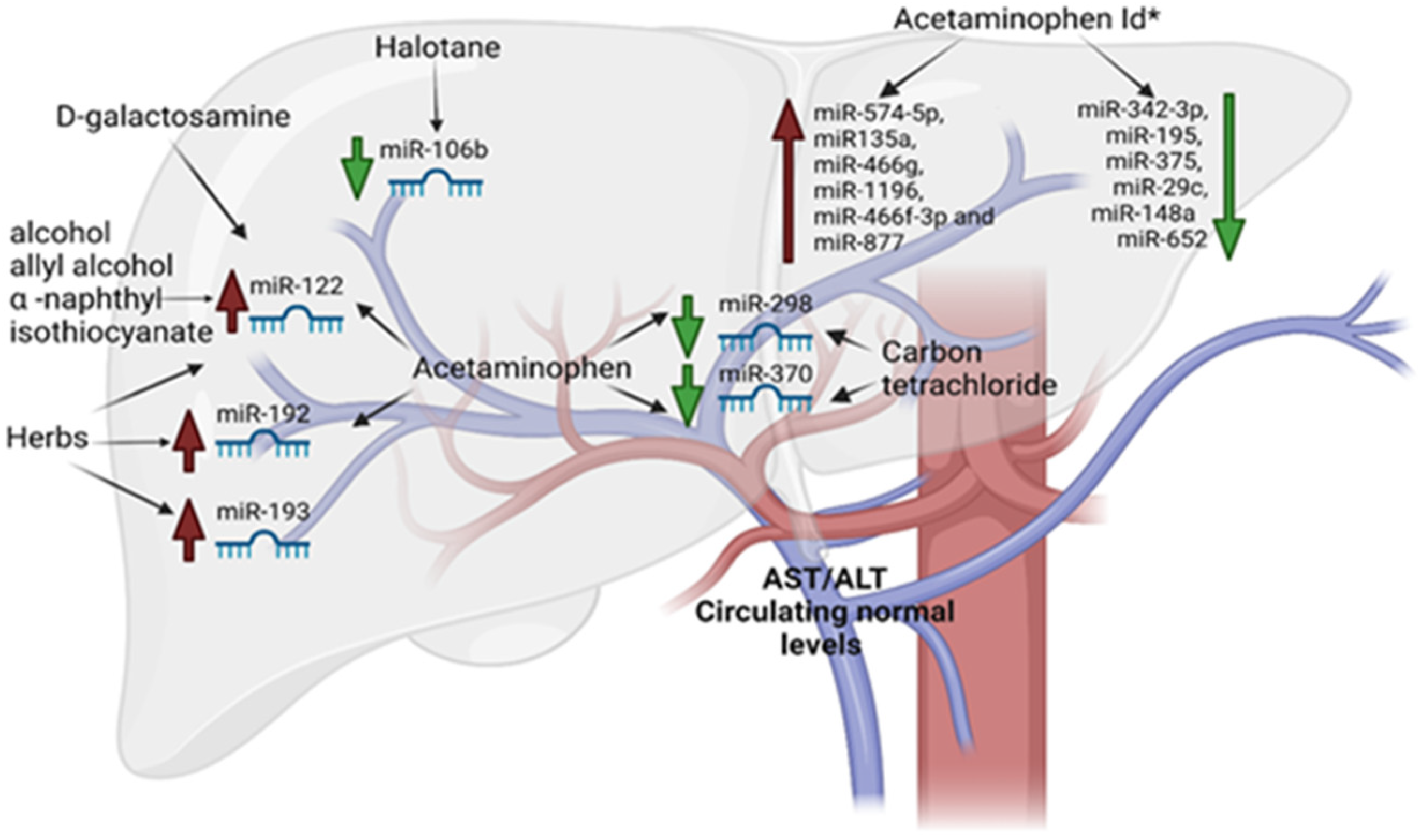
4. miRNAs in DILI
5. Hepatitis B Virus: Genome of and Pathogenesis
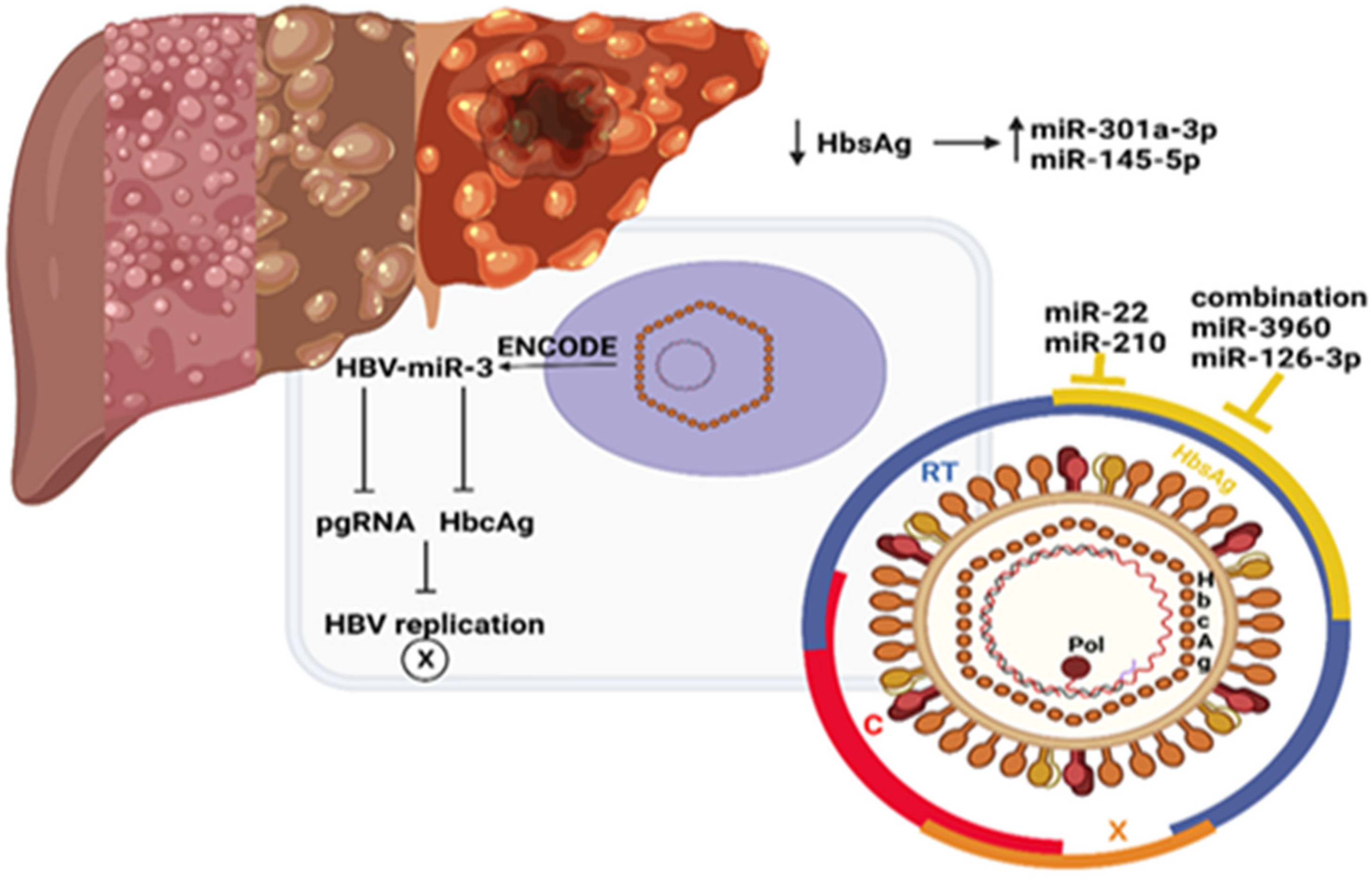
6. miRNAs and Hepatitis B Virus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter |
| ABCG1 | ATP Binding Cassette Subfamily G Member 1 |
| AKT | AKT serine/threonine kinase 1 |
| AMPK | AMP-activated protein kinase |
| Bmpr1a | bone morphogenetic protein receptor type 1A |
| CPT1α | Carnitine palmitoyltransferase I |
| CROT | Carnitine O-Octanoyltransferase |
| FOXA1 | Forkhead box protein A1 |
| G6PC | glucose-6-phosphatase |
| GSK | Glycogen Synthase Kinase |
| HAMP | Hepcidin Antimicrobial Peptide |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| HNF6 | hepatocyte nuclear factor 6 |
| HNFA4 | hepatocyte Nuclear Factor 4 Alpha |
| LXRα | liver X receptor alpha |
| mTOR | mammalian target of rapamycin |
| MTT | microsomal triglyceride transfer protein |
| NPC1 | NPC intracellular cholesterol transporter 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PIK3 | phosphatidylinositol-3-kinase |
| PTEN | Phosphatase and Tensin Homolog |
| SIRT1 | Sirtuin 1 |
| SIRT6 | Sirtuin 6 |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| VLDL | Very Low-Density Lipoprotein |
References
- Bénichou, C. Criteria of drug-induced liver disorders: Report of an International Consensus Meeting. J. Hepatol. 1990, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Bussières, J.-F.; Habra, M. Application of International Consensus Meeting Criteria for Classifying Drug-Induced Liver Disorders. Ann. Pharmacother. 1995, 29, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Orzeł-Gajowik, K.; Milewski, K.; Zielińska, M. Insight into microRNAs-Mediated Communication between Liver and Brain: A Possible Approach for Understanding Acute Liver Failure? Int. J. Mol. Sci. 2021, 23, 224. [Google Scholar] [CrossRef]
- Hand, N.J.; Master, Z.R.; Le Lay, J.; Friedman, J.R. Hepatic function is preserved in the absence of mature microRNAs. Hepatology 2009, 49, 618–626. [Google Scholar] [CrossRef]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Cione, E.; Cannataro, R.; Gallelli, L.; De Sarro, G.; Caroleo, M.C. Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals 2021, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Gatfield, D.; Le Martelot, G.; Vejnar, C.E.; Gerlach, D.; Schaad, O.; Fleury-Olela, F.; Ruskeepää, A.-L.; Oresic, M.; Esau, C.C.; Zdobnov, E.M.; et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009, 23, 1313–1326. [Google Scholar] [CrossRef]
- Castoldi, M.; Vujic Spasić, M.; Altamura, S.; Elmén, J.; Lindow, M.; Kiss, J.; Stolte, J.; Sparla, R.; D’Alessandro, L.A.; Klingmüller, U.; et al. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J. Clin. Investig. 2011, 121, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Zhang, X.; Fernández-Tussy, P.; Singh, A.K.; Burnap, S.A.; Rotllan, N.; Goedeke, L.; Sun, J.; Canfrán-Duque, A.; Aryal, B.; et al. Loss of hepatic miR-33 improves metabolic homeostasis and liver function without altering body weight or atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2006478118. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Ren, H.; Huang, X.; Shen, T.; Tang, W.; Dou, L.; Li, J. miR-23a/b-3p promotes hepatic lipid accumulation by regulating Srebp-1c and Fas. J. Mol. Endocrinol. 2021, 68, 35–49. [Google Scholar] [CrossRef]
- Zhong, D.; Huang, G.; Zhang, Y.; Zeng, Y.; Xu, Z.; Zhao, Y.; He, X.; He, F. MicroRNA-1 and microRNA-206 suppress LXRα-induced lipogenesis in hepatocytes. Cell. Signal. 2013, 25, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, C.; Qi, W.; Zhang, Y.; Zhang, F.; Wu, J.X.; Hu, Y.N.; Wu, D.M.; Liu, Y.; Yan, T.T.; et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia 2012, 55, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miu, K.-K.; Zhang, X.; Wan, A.T.-Y.; Lu, G.; Cheung, H.-H.; Lee, H.-M.; Kong, A.P.-S.; Chan, J.C.-N.; Chan, W.-Y. Hepatic miR-192-3p reactivation alleviates steatosis by targeting glucocorticoid receptor. JHEP Rep. 2020, 2, 100179. [Google Scholar] [CrossRef]
- Jia, Y.; Cong, R.; Li, R.; Yang, X.; Sun, Q.; Parvizi, N.; Zhao, R. Maternal Low-Protein Diet Induces Gender-Dependent Changes in Epigenetic Regulation of the Glucose-6-Phosphatase Gene in Newborn Piglet Liver. J. Nutr. 2012, 142, 1659–1665. [Google Scholar] [CrossRef]
- Dou, L.; Meng, X.; Sui, X.; Wang, S.; Shen, T.; Huang, X.; Guo, J.; Fang, W.; Man, Y.; Xi, J.; et al. MiR-19a regulates PTEN expression to mediate glycogen synthesis in hepatocytes. Sci. Rep. 2015, 5, 11602. [Google Scholar] [CrossRef]
- Azzimato, V.; Chen, P.; Barreby, E.; Morgantini, C.; Levi, L.; Vankova, A.; Jager, J.; Sulen, A.; Diotallevi, M.; Shen, J.X.; et al. Hepatic miR-144 Drives Fumarase Activity Preventing NRF2 Activation During Obesity. Gastroenterology 2021, 161, 1982–1997.e11. [Google Scholar] [CrossRef]
- Zhu, H.; Ng, S.-C.; Segrè, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 Axis Regulates Glucose Metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-G.; Qiu, R.-L.; Wu, Y.-H.; Li, Z.-X.; Xie, P.; Zhang, J.; Zhou, J.-J.; Zeng, L.-X.; Tang, J.; Maharjan, A.; et al. Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver Int. 2014, 34, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Zhu, L.-L.; Yang, X.-B.; Gao, N.; Fang, Y.; Wen, Q.; Qiao, H.-L. Variation in the expression of cytochrome P450-related miRNAs and transcriptional factors in human livers: Correlation with cytochrome P450 gene phenotypes. Toxicol. Appl. Pharmacol. 2021, 412, 115389. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Nakajima, M.; Mohri, T.; Yokoi, T. Post-transcriptional Regulation of Human Pregnane X Receptor by Micro-RNA Affects the Expression of Cytochrome P450 3A4. J. Biol. Chem. 2008, 283, 9674–9680. [Google Scholar] [CrossRef] [PubMed]
- Mohri, T.; Nakajima, M.; Fukami, T.; Takamiya, M.; Aoki, Y.; Yokoi, T. Human CYP2E1 is regulated by miR-378. Biochem. Pharmacol. 2010, 79, 1045–1052. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Surapureddi, S.; Coulter, S.; Ferguson, S.S.; Goldstein, J.A. Human CYP2C8 Is Post-Transcriptionally Regulated by MicroRNAs 103 and 107 in Human Liver. Mol. Pharmacol. 2012, 82, 529–540. [Google Scholar] [CrossRef]
- Takagi, S.; Nakajima, M.; Kida, K.; Yamaura, Y.; Fukami, T.; Yokoi, T. MicroRNAs Regulate Human Hepatocyte Nuclear Factor 4α, Modulating the Expression of Metabolic Enzymes and Cell Cycle. J. Biol. Chem. 2010, 285, 4415–4422. [Google Scholar] [CrossRef]
- Roh, Y.J.; Kim, Y.; Lee, J.S.; Oh, J.H.; Lee, S.M.; Yoon, E.L.; Lee, S.R.; Jun, D.W. Regulation of Hepatocyte Nuclear Factor 4α Attenuated Lipotoxicity but Increased Bile Acid Toxicity in Non-Alcoholic Fatty Liver Disease. Life 2022, 12, 1682. [Google Scholar] [CrossRef]
- Takahashi, K.; Oda, Y.; Toyoda, Y.; Fukami, T.; Yokoi, T.; Nakajima, M. Regulation of Cytochrome b 5 Expression by miR-223 in Human Liver: Effects on Cytochrome P450 Activities. Pharm. Res. 2014, 31, 780–794. [Google Scholar] [CrossRef]
- Sgro, C.; Clinard, F.; Ouazir, K.; Chanay, H.; Allard, C.; Guilleminet, C.; Lenoir, C.; Lemoine, A.; Hillon, P. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002, 36, 451–455. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, Presentation, and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterol. 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Liangpunsakul, S.; Chalasani, N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am. J. Gastroenterol. 2007, 102, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Koch, D.G.; Lee, W.M. Druginduced acute liver failure: Results of a U.S. multicenter, prospective study. Hepatology 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Bergquist, A.; Broomé, U.; Lindgren, S.; Wallerstedt, S.; Almer, S.; Sangfelt, P.; Danielsson, H.; Sandberg-Gertzén, H.; Lööf, L.; et al. Acute liver failure in Sweden: Etiology and outcome. J. Intern. Med. 2007, 262, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. MicroRNA (Shariqah, United Arab Emirates) 2019, 8, 116–126. [Google Scholar] [CrossRef]
- Lu, R.-J.; Zhang, Y.; Tang, F.-L.; Zheng, Z.-W.; Fan, Z.-D.; Zhu, S.-M.; Qian, X.-F.; Liu, N.-N. Clinical characteristics of drug-induced liver injury and related risk factors. Exp. Ther. Med. 2016, 12, 2606–2616. [Google Scholar] [CrossRef]
- Koch, L. Propylthiouracil use associated with severe hepatotoxicity in children. Nat. Rev. Endocrinol. 2010, 6, 416. [Google Scholar] [CrossRef]
- Cortes, M.G.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Causes, Clinical Features, and Outcomes from a Prospective Study of Drug-Induced Liver Injury in the United States. Gastroenterology 2008, 135, 1924–1934.e4. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Maddur, H.; Russo, M.W.; Wong, R.J.; Reddy, K.R. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2021, 116, 878–898. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin. Pharmacokinet. 2021, 60, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Davern, T.J. Drug-Induced Liver Disease. Clin. Liver Dis. 2012, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.J. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd ed; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients with Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e7. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.D.; Abu-Sbeih, H.; Styskel, B.; Gonzalez, G.M.N.; Blechacz, B.; Naing, A.; Chalasani, N. Clinical Characteristics and Adverse Impact of Hepatotoxicity due to Immune Checkpoint Inhibitors. Am. J. Gastroenterol. 2020, 115, 251–261. [Google Scholar] [CrossRef]
- O’Donnell, J.T.; Marks, D.H.; Danese, P.; O’Donnell, J.J., III. Drug-induced liver disease: Primer for the primary care physician. Disease-A-Month 2014, 60, 55–104. [Google Scholar] [CrossRef]
- Holt, M.P.; Ju, C. Mechanisms of drug-induced liver injury. AAPS J. 2006, 8, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaplowitz, N. Mechanisms of Drug-induced Liver Injury. Clin. Liver Dis. 2013, 17, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Obermayer-Straub, P.; Manns, M.P. Immunological mechanisms in liver injury. In Drug-Induced Liver Disease; Kaplowitz, N., DeLeve, L., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 125–149. [Google Scholar]
- Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005, 4, 489–499. [Google Scholar] [CrossRef]
- Abboud, G.; Kaplowitz, N. Drug-induced liver injury. Drug Saf. 2007, 30, 277–294. [Google Scholar] [CrossRef]
- Sakaan, S.A.; Twilla, J.D.; Usery, J.B.; Winton, J.C.; Self, T.H. Nitrofurantoin-Induced Hepatotoxicity: A Rare Yet Serious Complication. South. Med. J. 2014, 107, 107–113. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a Prospective Study of Acute Liver Failure at 17 Tertiary Care Centers in the United States. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Acetaminophen hepatotoxicity: NO to the rescue. J. Cereb. Blood Flow Metab. 2004, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kozer, E.; Koren, G. Management of paracetamol overdose: Current controversies. Drug Saf. 2001, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Pye, M.; Northcote, R.J.; Cobbe, S.M. Acute hepatitis after parenteral amiodarone administration. Heart 1988, 59, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.; Reisch, J.S.; Schiødt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.A.; Berry, R.S. Hepatotoxicity associated with sustained-release niacin. Am. J. Med. 1992, 93, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; Martin-Vivaldi, R. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Schmeltzer, P.A.; Kosinski, A.S.; Kleiner, D.E.; Hoofnagle, J.H.; Stolz, A.; Fontana, R.J.; Russo, M.W.; Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016, 36, 603–609. [Google Scholar] [CrossRef]
- de Boer, Y.S.; Kosinski, A.S.; Urban, T.J.; Zhao, Z.; Long, N.; Chalasani, N.; Drug-Induced Liver Injury Network. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin. Gastroenterol. Hepatol. 2017, 15, 103–112.e2. [Google Scholar] [CrossRef]
- Ahmad, J.; Odin, J.A.; Hayashi, P.H.; Chalasani, N.; Fontana, R.J.; Barnhart, H.; Cirulli, E.T.; Kleiner, D.E.; Hoofnagle, J.H. Identification and Characterization of Fenofibrate-Induced Liver Injury. Am. J. Dig. Dis. 2017, 62, 3596–3604. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Kleiner, D.E.; Ghabril, M.; Gu, J.; Hoofnagle, J.H.; Rockey, D.C. Identification and Characterization of Cefazolin-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2015, 13, 1328–1336.e2. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J.; Cirulli, E.T.; Gu, J.; Kleiner, D.; Ostrov, D.; Phillips, E.; Schutte, R.; Barnhart, H.; Chalasani, N.; Watkins, P.B.; et al. The role of HLA-A*33:01 in patients with cholestatic hepatitis attributed to terbinafine. J. Hepatol. 2018, 69, 1317–1325. [Google Scholar] [CrossRef]
- Grant, L.M.; Kleiner, D.E.; Conjeevaram, H.S.; Vuppalanchi, R.; Lee, W.M. Clinical and Histological Features of Idiosyncratic Acute Liver Injury Caused by Temozolomide. Am. J. Dig. Dis. 2013, 58, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Orman, E.; Conjeevaram, H.S.; Vuppalanchi, R.; Freston, J.W.; Rochon, J.; Kleiner, D.; Hayashi, P.H. Clinical and Histopathologic Features of Fluoroquinolone-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2011, 9, 517–523.e3. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A.; Vuppalanchi, R.; Fontana, R.J.; Stolz, A.; Kleiner, D.E.; Hayashi, P.H.; Gu, J.; Hoofnagle, J.H.; Chalasani, N. Clinical and Histologic Features of Azithromycin-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2015, 13, 369–376.e3. [Google Scholar] [CrossRef] [PubMed]
- Ghabril, M.; Bonkovsky, H.L.; Kum, C.; Davern, T.; Hayashi, P.H.; Kleiner, D.; Serrano, J.; Rochon, J.; Fontana, R.J.; Bonacini, M. Liver Injury From Tumor Necrosis Factor-α Antagonists: Analysis of Thirty-four Cases. Clin. Gastroenterol. Hepatol. 2013, 11, 558–564.e3. [Google Scholar] [CrossRef]
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.X.; Rossi, S.; Fontana, R.J.; Vuppalanchi, R.; Hoofnagle, J.H.; Khan, I.; Navarro, V.J. Risk of Liver Injury Associated with Green Tea Extract in SLIMQUICK® Weight Loss Products: Results from the DILIN Prospective Study. Drug Saf. 2016, 39, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, S.; Marzolf, B.; Troisch, P.; Brightman, A.; Hu, Z.; Hood, L.E.; Galas, D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA 2009, 106, 4402–4407. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Zheng, R.; Guo, Y.; Wang, Y.; Guo, H.; Fei, M.; Sun, S. Plasma MicroRNA-122 as a Biomarker for Viral-, Alcohol-, and Chemical-Related Hepatic Diseases. Clin. Chem. 2010, 56, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Petrasek, J.; Mundkur, S.; Catalano, D.; Levin, I.; Ward, J.; Alao, H.; Kodys, K.; Szabo, G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012, 56, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Bala, S.; Petrasek, J.; Szabo, G. Plasma microRNA profiles distinguish lethal injury in acetaminophen toxicity: A research study. World J. Gastroenterol. 2012, 18, 2798–2804. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, X.; Mendrick, D.L. Hopes and challenges in using miRNAs as translational biomarkers for drug-induced liver injury. Biomarkers Med. 2013, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, Y.; Nakajima, M.; Takagi, S.; Fukami, T.; Tsuneyama, K.; Yokoi, T. Plasma MicroRNA Profiles in Rat Models of Hepatocellular Injury, Cholestasis, and Steatosis. PLoS ONE 2012, 7, e30250. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-W.; Chen, X.; Jiang, Z.-Z.; Wang, T.; Wang, C.; Zhang, Y.; Wen, J.; Xue, M.; Zhu, D.; Zhang, Y.; et al. A Panel of Serum MicroRNAs as Specific Biomarkers for Diagnosis of Compound- and Herb-Induced Liver Injury in Rats. PLoS ONE 2012, 7, e37395. [Google Scholar] [CrossRef]
- Starckx, S.; Batheja, A.; Verheyen, G.R.; De Jonghe, S.; Steemans, K.; Van Dijck, B.; Singer, M.; Bogdan, N.; Snoeys, J.; Vinken, P.; et al. Evaluation of miR-122 and Other Biomarkers in Distinct Acute Liver Injury in Rats. Toxicol. Pathol. 2013, 41, 795–804. [Google Scholar] [CrossRef]
- Lewis, P.J.S.; Dear, J.; Platt, V.; Simpson, K.J.; Craig, D.G.; Antoine, D.J.; French, N.; Dhaun, N.; Webb, D.J.; Costello, E.; et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology 2011, 54, 1767–1776. [Google Scholar] [CrossRef]
- Jetten, M.J.; Gaj, S.; Ruiz-Aracama, A.; de Kok, T.M.; van Delft, J.H.; Lommen, A.; van Someren, E.P.; Jennen, D.G.; Claessen, S.M.; Peijnenburg, A.A.; et al. Omics analysis of low dose acetaminophen intake demonstrates novel response pathways in humans. Toxicol. Appl. Pharmacol. 2012, 259, 320–328. [Google Scholar] [CrossRef]
- Antoine, D.J.; Dear, J.W.; Lewis, P.S.; Platt, V.; Coyle, J.; Masson, M.; Thanacoody, R.H.; Gray, A.J.; Webb, D.J.; Moggs, J.G.; et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology 2013, 58, 777–787. [Google Scholar] [CrossRef]
- Ding, J.; Ding, X.; Ning, J.; Yi, F.; Chen, J.; Zhao, D.; Zheng, J.; Liang, Z.; Hu, Z.; Du, Q. Circulating microRNA-122 as a potential biomarker for liver injury. Mol. Med. Rep. 2012, 5, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.S.; Lutwick, L.J. The virus of hepatitis type B. N. Engl. J. Med. 1976, 295, 1232–1236. [Google Scholar] [CrossRef]
- Miller, R.H.; Kaneko, S.; Chung, C.T.; Girones, R.; Purcell, R.H. Compact organization of the hepatitis B virus genome. Hepatology 1989, 9, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Heermann, K.H.; Goldmann, U.; Schwartz, W.; Seyffarth, T.; Baumgarten, H.; Gerlich, W.H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 1984, 52, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, W. Hepatitis B surface proteins. J. Hepatol. 1991, 13, S90–S92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Zhang, Y.; Xu, M.; Li, X.; Zhang, Z. Distribution of hepatitis B virus genotypes and subgenotypes: A meta-analysis. Medicine 2021, 100, e27941. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Hepatitis B Virus Biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51–68. [Google Scholar] [CrossRef]
- Radziwill, G.; Tucker, W.; Schaller, H. Mutational analysis of the hepatitis B virus P gene product: Domain structure and RNase H activity. J. Virol. 1990, 64, 613–620. [Google Scholar] [CrossRef]
- Ganem, D.; Varmus, H.E. The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 1987, 56, 651–693. [Google Scholar] [CrossRef]
- Alexander, G.J.M. Immunology of hepatitis B virus infection. Br. Med Bull. 1990, 46, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Edgington, T.S.; Chisari, F. Immunological aspects of hepatitis B virus infection. Am. J. Med Sci. 1975, 270, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Penna, A.; Bertoletti, A.; Valli, A.; Antoni, A.D.; Giuberti, T.; Cavalli, A.; Petit, M.A.; Fiaccadori, F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 1990, 145, 3442–3449. [Google Scholar] [PubMed]
- Ferrari, C.; Penna, A.; DegliAntoni, A.; Fiaccadori, F. Cellular immune response to hepatitis B virus antigens: An overview. J. Hepatol. 1988, 7, 21–33. [Google Scholar] [CrossRef]
- Chisari, F.; Ferrari, C.; Mondelli, M. Hepatitis B virus structure and biology. Microb. Pathog. 1989, 6, 311–325. [Google Scholar] [CrossRef]
- Li, M.H.; Lu, Y.; Zhang, L.; Wang, X.Y.; Ran, C.P.; Hao, H.X.; Xie, Y. Association of Cytokines with Alanine Aminotransferase, Hepatitis B Virus Surface Antigen and Hepatitis B Envelope Antigen Levels in Chronic Hepatitis B. Chin. Med. J. 2018, 131, 1813–1818. [Google Scholar] [CrossRef]
- Moroni, M.; Esposito, R.; De Lalla, F. Malattie Infettive; Masson: Milan, Italy, 2003; p. 541. [Google Scholar]
- Trépo, C.; Chan, H.L.Y.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, W.; Wang, Y.; Qiao, L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014, 345, 216–222. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Sun, H.; Fan, H.; Hu, Y.; Liu, M.; Li, X.; Tang, H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J. Virol. 2017, 91, e01919-16. [Google Scholar] [CrossRef]
- Iacob, D.G.; Rosca, A.; Ruta, S. Circulating microRNAs as non-invasive biomarkers for hepatitis B virus liver fibrosis. World J. Gastroenterol. 2020, 26, 1113–1127. [Google Scholar] [CrossRef]
- van der Ree, M.H.; Jansen, L.; Kruize, Z.; van Nuenen, A.C.; van Dort, K.A.; Takkenberg, R.B.; Reesink, H.W.; Kootstra, N.A. Plasma MicroRNA Levels Are Associated with Hepatitis B e Antigen Status and Treatment Response in Chronic Hepatitis B Patients. J. Infect. Dis. 2017, 215, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, M.; Deng, Y.; Guo, Y.; Zhang, X.; Xiang, D.; Jiang, L.; You, Z.; Wu, Y.; Li, M.; et al. Pretreatment microRNA levels can predict HBsAg clearance in CHB patients treated with pegylated interferon α-2a. Virol. J. 2018, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, C.; Wu, M.; Chen, L.; Zhang, J.; Zhang, X.; Zhang, Z.; Wu, J.; Wang, J.; Chen, X.; et al. Plasma Microrna Profile as a Predictor of Early Virological Response to Interferon Treatment in Chronic Hepatitis B Patients. Antivir. Ther. 2012, 17, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, M.R.; Cavallone, D.; Oliveri, F.; Moriconi, F.; Colombatto, P.; Coco, B.; Ciccorossi, P.; Rastelli, C.; Romagnoli, V.; Cherubini, B.; et al. A Serum MicroRNA Signature Is Associated with the Immune Control of Chronic Hepatitis B Virus Infection. PLoS ONE 2014, 9, e110782. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, X.; Chen, L.; Zhang, Z.; Zhang, J.; Wang, W.; Wu, M.; Shi, B.; Zhang, X.; Kozlowski, M.; et al. Circulating miR-210 and miR-22 combined with ALT predict the virological response to interferon-alpha therapy of CHB patients. Sci. Rep. 2017, 7, 15658. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef]
| Drugs | DILI |
|---|---|
| Checkpoint inhibitors, Immunomodulatory MABs | Indirect hepatotoxicity |
| Tetracycline, Valproic acid, Tamoxifene | Mitochondrial dysfunction |
| Nitrofurantoin, Sulfonamides, Erythromycin, Amoxicillin-clavulanate | Immune-mediated idiosyncratic DILI |
| Metotrexate, Amiodarone | Liver fibrosis, Cirrosis |
| Acetaminophen, Aspirin, Niacin, Antineoplastic agents | Acute hepatic necrosis |
| Isoniazid, Nitrofurantoin, Diclofenac | Acute hepatocellular hepatitis |
| Nitrofurantoin, Minocycline, Hydralazine, Methyldopa, Statins, Fenofibrate | Autoimmune-like chronic liver injury |
| Amoxicillin–clavulanate, Cephalosporins, Terbinafine, Azathioprine, Temozolomide | Cholestatic liver injury |
| Fluoroquinolone and Macrolide antibiotics, Phenytoin, Sulfonamides | Mixed hepatitis |
| Imatinib, Nilotinib, Bortezomib, Pazopanib, Ribociclib | Jaundice (rare) |
| Green tea extracts | Acute hepatocellular hepatitis |
| Drugs and Chemicals | microRNA Upregulated | microRNA Downregulated |
|---|---|---|
| D-galactosamine | miR-122 | |
| Tamoxifen | miR-106a, 17–92 cluster, and miR-34 | |
| Acetaminophen | miR-122 and miR-192 | miR-298 and miR-370 |
| Acetaminophen ld * | miR-1196, miR135a, miR-466f-3p, miR-466g, miR-877 and miR-574-5p | miR342-3p, miR-195, miR-375, miR-29c, miR-148a and miR-652 |
| Alcohol | miR-122 | |
| Allyl alcohol | miR-122 | |
| α -naphthyl isothiocyanate | miR-122 | |
| Carbon tetrachloride | miR-298 and miR-370 | |
| Halothane | miR-106b | |
| Herbs | miR-193, miR-192, and miR-122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cione, E.; Abrego Guandique, D.M.; Caroleo, M.C.; Luciani, F.; Colosimo, M.; Cannataro, R. Liver Damage and microRNAs: An Update. Curr. Issues Mol. Biol. 2023, 45, 78-91. https://doi.org/10.3390/cimb45010006
Cione E, Abrego Guandique DM, Caroleo MC, Luciani F, Colosimo M, Cannataro R. Liver Damage and microRNAs: An Update. Current Issues in Molecular Biology. 2023; 45(1):78-91. https://doi.org/10.3390/cimb45010006
Chicago/Turabian StyleCione, Erika, Diana Marisol Abrego Guandique, Maria Cristina Caroleo, Filippo Luciani, Manuela Colosimo, and Roberto Cannataro. 2023. "Liver Damage and microRNAs: An Update" Current Issues in Molecular Biology 45, no. 1: 78-91. https://doi.org/10.3390/cimb45010006
APA StyleCione, E., Abrego Guandique, D. M., Caroleo, M. C., Luciani, F., Colosimo, M., & Cannataro, R. (2023). Liver Damage and microRNAs: An Update. Current Issues in Molecular Biology, 45(1), 78-91. https://doi.org/10.3390/cimb45010006









