The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis
Abstract
1. Introduction
2. Oxidative Stress and Inflammation
3. RBCs and Oxidative Stress
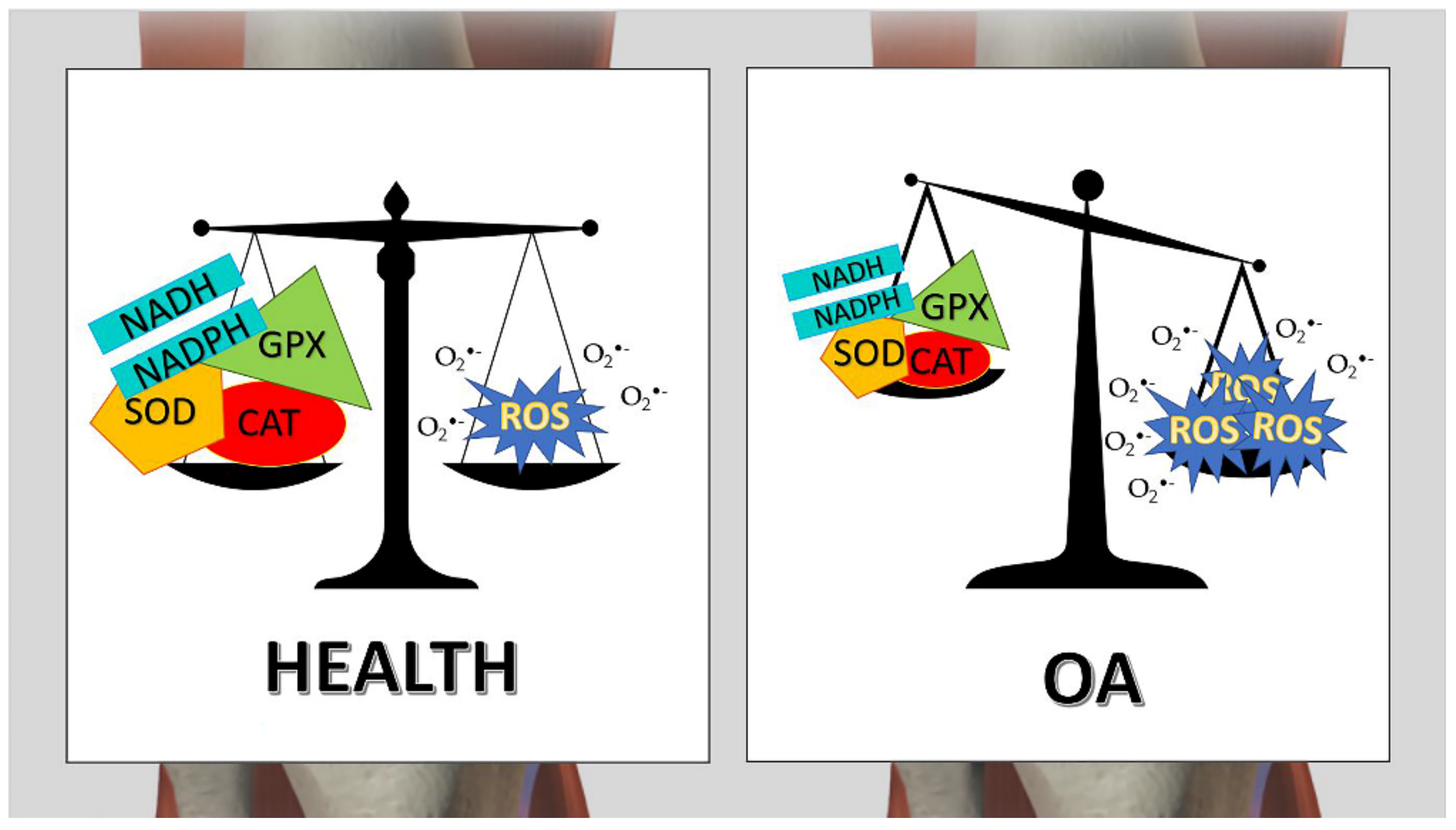
4. Osteoarthritis and RBCs Oxidative Stress
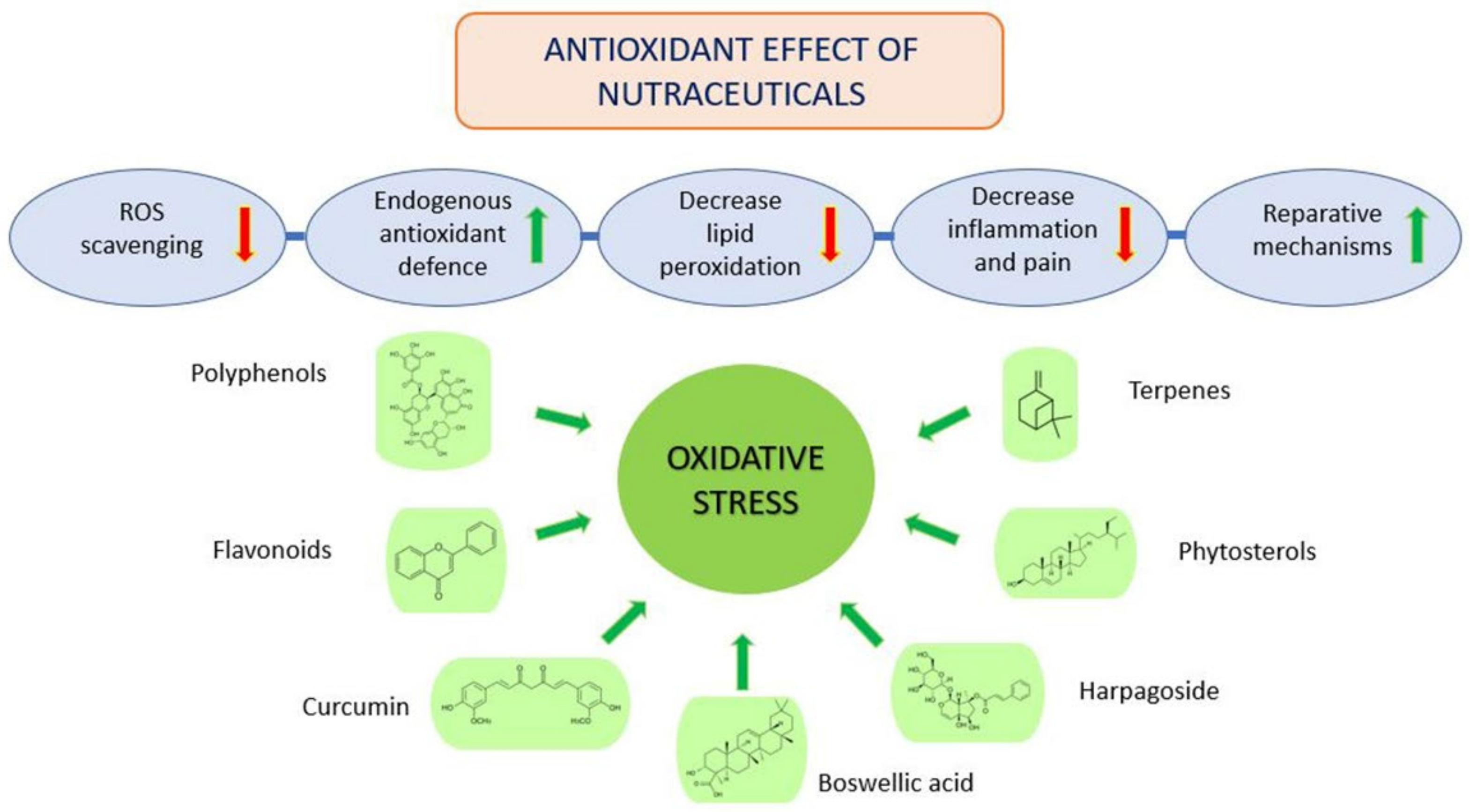
5. Osteoarthritis and Antioxidant Treatment
5.1. Harpagophytum Procumbens
5.2. Boswellia Serrata
5.3. Curcuma Longa
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and incidence of osteoarthritis: A population-based retrospective cohort study. J. Clin. Med. 2021, 10, 4282. [Google Scholar] [CrossRef] [PubMed]
- Miehle, W. Arthrosis or osteoarthritis: Do these terms imply therapy with pure analgesics or non-steroidal antirheumatic agents? Scand. J. Rheumatol. 1987, 16, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Gouttebarge, V.; Inklaar, H.; Backx, F.; Kerkhoffs, G. Prevalence of osteoarthritis in former elite athletes: A systematic overview of the recent literature. Rheumatol. Int. 2015, 35, 405–418. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Scotto d’Abusco, A.; Corsi, A.; Grillo, M.G.; Cicione, C.; Calamia, V.; Panzini, G.; Sansone, A.; Giordano, C.; Politi, L.; Scandurra, R. Effects of intra-articular administration of glucosamine and a peptidyl-glucosamine derivative in a rabbit model of experimental osteoarthritis: A pilot study. Rheumatol. Int. 2008, 28, 437–443. [Google Scholar] [CrossRef]
- Honvo, G.; Reginster, J.Y.; Rabenda, V.; Geerinck, A.; Mkinsi, O.; Charles, A.; Rizzoli, R.; Cooper, C.; Avouac, B.; Bruyère, O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 65–99. [Google Scholar] [CrossRef]
- Jerosch, J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef]
- Uitterlinden, E.; Koevoet, J.; Verkoelen, C.; Bierma-Zeinstra, S.; Jahr, H.; Weinans, H.; Verhaar, J.; van Osch, G. Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants. BMC Musculoskelet. Disord. 2008, 9, 120. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; Peroza, L.R.; Boligon, A.A.; Athayde, M.L.; Alves, S.H.; Fachinetto, R.; Wagner, C. Harpagophytum procumbens Prevents Oxidative Stress and Loss of Cell Viability In Vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, A.F.; El-Kazaz, S.E.; Noreldin, A.E. Boswellia serrata suppress fipronil-induced neuronal necrosis and neurobehavioral alterations via promoted inhibition of oxidative/inflammatory/apoptotic pathways. Sci. Total Environ. 2021, 785, 147384. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e381. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin inhibits the PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid. Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Zhu, S.; Makosa, D.; Miller, B.; Griffin, T.M. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect. Tissue Res. 2020, 61, 34–47. [Google Scholar] [CrossRef]
- Davies, C.M.; Guilak, F.; Weinberg, J.B.; Fermor, B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthr. Cartil. 2008, 16, 624–630. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Pimlott, Z.; Hontoir, F.; Kharaz, Y.A.; Anderson, J.; Dyer, P.; Collins, J.; Loeser, R.; Welting, T.; Caron, M.; Peffers, M.J. Small nucleolar RNAs as mediators of oxidative stress in cross species cartilage and osteoarthritis. Osteoarthr. Cartil. 2020, 28, s342. [Google Scholar] [CrossRef]
- Idzik, M.; Poloczek, J.; Skrzep-Poloczek, B.; Chełmecka, E.; Jochem, J.; Stygar, D. General Rehabilitation Program after Knee or Hip Replacement Significantly Influences Erythrocytes Oxidative Stress Markers and Serum ST2 Levels. Oxid. Med. Cell. Longev. 2022, 2022, 1358858. [Google Scholar] [CrossRef] [PubMed]
- Jabri, M.-A.; Sani, M.; Rtibi, K.; Marzouki, L.; El-Benna, J.; Sakly, M.; Sebai, H. Chamomile decoction extract inhibits human neutrophils ROS production and attenuates alcohol-induced haematological parameters changes and erythrocytes oxidative stress in rat. Lipids Health Dis. 2016, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef]
- Sen Gupta, A. Hemoglobin-based Oxygen Carriers: Current State-of-the-art and Novel Molecules. Shock 2019, 52, 70–83. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Biomarkers of oxidative stress in red blood cells. Biomed. Pap. 2011, 155, 131–136. [Google Scholar] [CrossRef]
- Li, H.; Lykotrafitis, G. Erythrocyte membrane model with explicit description of the lipid bilayer and the spectrin network. Biophys. J. 2014, 107, 624–653. [Google Scholar] [CrossRef]
- Narla, J.; Mohandas, N. Red cell membrane disorders. Int. J. Lab. Hematol. 2017, 39, 47–52. [Google Scholar] [CrossRef]
- Faivre, B.; Menu, P.; Labrude, P.; Vigneron, C. Hemoglobin Autooxidation/Oxidation Mechanisms and Methemoglobin Prevention or Reduction Processes in the Bloodstream Literature review and outline of autooxidation reaction. Artif. Cells Blood Substit. Biotechnol. 1998, 26, 17–26. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216–222. [Google Scholar] [CrossRef]
- Hill, S.; Lamberson, C.R.; Xu, L.; To, R.; Tsui, H.S.; Shmanai, V.V.; Bekish, A.V.; Awad, A.M.; Marbois, B.N.; Cantor, C.R.; et al. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic. Biol. Med. 2012, 53, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation—DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Markers of Oxidative Stress in Erythrocytes and Plasma During Aging in Humans. Oxid. Med. Cell. Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Terrill, J.R.; Radley-Crabb, H.G.; Iwasaki, T.; Lemckert, F.A.; Arthur, P.G.; Grounds, M.D. Oxidative stress and pathology in muscular dystrophies: Focus on protein thiol oxidation and dysferlinopathies. FEBS J. 2013, 280, 4149–4164. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohkubo, N.; Aoto, M.; Maeda, N.; Cicha, I.; Miki, T.; Mitsuda, N. Participation of caspase-3-like protease in oxidation-induced impairment of erythrocyte membrane properties. Biorheology 2007, 44, 179–190. [Google Scholar]
- Carelli-Alinovi, C.; Ficarra, S.; Russo, A.M.; Giunta, E.; Barreca, D.; Galtieri, A.; Misiti, F.; Tellone, E. Involvement of acetylcholinesterase and protein kinase C in the protective effect of caffeine against β-amyloid-induced alterations in red blood cells. Biochimie 2016, 121, 52–59. [Google Scholar] [CrossRef]
- Misiti, F.; Orsini, F.; Clementi, M.E.; Masala, D.; Tellone, E.; Galtieri, A.; Giardina, B. Amyloid peptide inhibits ATP release from human erythrocytes. Biochem. Cell Biol. 2008, 86, 501–508. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Misiti, F. Erythrocytes as Potential Link between Diabetes and Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Tellone, E.; Giardina, B.; Scatena, R.; Russo, A.; Misiti, F.; Clementi, M.E.; Colucci, D.; Bellocco, E.; Laganà, G.; et al. Derangement of Erythrocytic AE1 in Beta-Thalassemia by Caspase 3: Pathogenic Mechanisms and Implications in Red Blood Cell Senescence. J. Membr. Biol. 2009, 228, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Deyhim, M.R.; Navabi, Z.; Jalili, M.A.; Maghsoudloo, M.; Khoshnaghsh, F. Alternation in erythrocyte enzyme antioxidant activity during blood storage. Iran. J. Blood Cancer 2014, 6, 69–74. [Google Scholar]
- Asakura, H.; Kitahora, T. Antioxidants and Polyphenols in Inflammatory Bowel Disease: Ulcerative Colitis and Crohn Disease. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–292. [Google Scholar]
- Chang, J.C.; van der Hoeven, L.H.; Haddox, C.H. Glutathione reductase in the red blood cells. Ann. Clin. Lab. Sci. 1978, 8, 23–29. [Google Scholar] [CrossRef]
- Zachara, B.A.; Gromadzińska, J.; Wasowicz, W.; Zbróg, Z. Red blood cell and plasma glutathione peroxidase activities and selenium concentration in patients with chronic kidney disease: A review. Acta Biochim. Pol. 2006, 53, 663–677. [Google Scholar] [CrossRef]
- Colombo, G.; Rossi, R.; Gagliano, N.; Portinaro, N.; Clerici, M.; Annibal, A.; Giustarini, D.; Colombo, R.; Milzani, A.; Dalle-Donne, I. Red Blood Cells Protect Albumin from Cigarette Smoke–Induced Oxidation. PLoS ONE 2012, 7, e29930. [Google Scholar] [CrossRef]
- Melo, D.; Rocha, S.; Coimbra, S.; Santos Silva, A. Interplay between Erythrocyte Peroxidases and Membrane. In Erythrocyte; IntechOpen: London, UK, 2019. [Google Scholar]
- Knight, J.A.; Blaylock, R.C.; Searles, D.A. The effect of vitamins C and E on lipid peroxidation in stored erythrocytes. Ann. Clin. Lab. Sci. 1993, 23, 51–56. [Google Scholar] [PubMed]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Biemond, P.; Swaak, A.J.G.; Koster, J.F. Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 1984, 27, 760–765. [Google Scholar] [CrossRef]
- Shin, D.M.; Moon, Y.R.; Lee, B.R. Superoxide Dismutase, Catalase and Glutathione Peroxidase Activities in Erythrocytes and Synovial Fluid of the Osteoarthritis of the Knee Joint. J. Korean Orthop. Assoc. 1994, 29, 44–49. [Google Scholar] [CrossRef]
- Maneesh, M.; Jayalekshmi, H.; Suma, T.; Chatterjee, S.; Chakrabarti, A.; Singh, T.A. Evidence for oxidative stress in osteoarthritis. Indian J. Clin. Biochem. 2005, 20, 129–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Gambhir, J.K.; Lali, P.; Jain, A.K. Correlation between blood antioxidant levels and lipid peroxidation in rheumatoid arthritis. Clin. Biochem. 1997, 30, 351–355. [Google Scholar] [CrossRef]
- Surapaneni, K.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007, 61, 9–14. [Google Scholar] [CrossRef]
- Halsted, C.H. Dietary supplements and functional foods: 2 sides of a coin? Am. J. Clin. Nutr. 2003, 77, 1001S–1007S. [Google Scholar] [CrossRef]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef]
- Acamovic, T.; Brooker, J.D. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 2005, 64, 403–412. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Terpenes and Terpenoids: Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef]
- Frank, L.; Wenig, M.; Ghirardo, A.; Krol, A.; Vlot, A.C.; Schnitzler, J.; Rosenkranz, M. Isoprene and β-caryophyllene confer plant resistance via different plant internal signalling pathways. Plant Cell Environ. 2021, 44, 1151–1164. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as Plant Antioxidants. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2005; Volume 72, pp. 505–535. [Google Scholar]
- Qi, J.; Chen, J.-J.; Cheng, Z.-H.; Zhou, J.-H.; Yu, B.-Y.; Qiu, S.X. Iridoid glycosides from Harpagophytum procumbens D.C. (devil’s claw). Phytochemistry 2006, 67, 1372–1377. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef]
- Hostanska, K.; Melzer, J.; Rostock, M.; Suter, A.; Saller, R. Alteration of anti-inflammatory activity of Harpagophytum procumbens (devil’s claw) extract after external metabolic activation with S9 mix. J. Pharm. Pharmacol. 2014, 66, 1606–1614. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; Hansen, C.; Shakibaei, M. Wirkung des Extraktes aus Harpagophytum procumbens DC auf Matrix-Metalloproteinasen in menschlichen Knorpelzellen in vitro. Arzneimittelforschung 2011, 54, 213–220. [Google Scholar] [CrossRef]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Di Maio, V.; Gulli, M.; Vedova, P.D.; Ammendola, S.; D’Abusco, A.S. Antiarthritic effects of a root extract from harpagophytum procumbens DC: Novel insights into the molecular mechanisms and possible bioactive phytochemicals. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef]
- Mariano, A.; Bigioni, I.; Mattioli, R.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Mariani, P.F.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals 2022, 15, 457. [Google Scholar] [CrossRef]
- Farpour, H.R.; Rajabi, N.; Ebrahimi, B. The Efficacy of Harpagophytum procumbens (Teltonal) in Patients with Knee Osteoarthritis: A Randomized Active-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2021, 2021, 5596892. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Harpagophytum procumbens DC. and/or Harpagophytum zeyheri Decne., radix; EMA/HMPC/627058/2015; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Peruru, R.; Usha Rani, R.; Thatiparthi, J.; Sampathi, S.; Dodoala, S.; Prasad, K.V.S.R.G. Devil’s claw (Harpagophytum procumbens) ameliorates the neurobehavioral changes and neurotoxicity in female rats exposed to arsenic. Heliyon 2020, 6, e03921. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bhattcharya, S.K. Anti-oxidant activity of Harpagophytum procumbens (devil’s claw). Br. J. Phyther. 1998, 5, 68–71. [Google Scholar]
- Georgiev, M.; Alipieva, K.; Pashova, S.; Denev, P.; Angelova, M.; Kerns, G.; Bley, T. Antioxidant activity of devil’s claw cell biomass and its active constituents. Food Chem. 2010, 121, 967–972. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Alipieva, K.I.; Denev, P. Antioxidant Activity and Bioactive Constituents of the Aerial Parts of Harpagophytum procumbens Plants. Biotechnol. Biotechnol. Equip. 2010, 24, 438–443. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Bertocchi, M.; Isani, G.; Medici, F.; Andreani, G.; Tubon Usca, I.; Roncada, P.; Forni, M.; Bernardini, C. Anti-Inflammatory Activity of Boswellia serrata Extracts: An In Vitro Study on Porcine Aortic Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 2504305. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phyther. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Sengupta, K.; Alluri, K.V.; Satish, A.; Mishra, S.; Golakoti, T.; Sarma, K.V.S.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef] [PubMed]
- Thawani, V.; Pimpalkhute, S.; Kabra, P.; Babhulkar, S.; Hingorani, L.; Sontakke, S. Open, randomized, controlled clinical trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian J. Pharmacol. 2007, 39, 27–29. [Google Scholar] [CrossRef]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee—A randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7. [Google Scholar] [CrossRef]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-Keto-β-Boswellic Acid Potentiates Apoptosis, Inhibits Invasion, and Abolishes Osteoclastogenesis by Suppressing NF-κB and NF-κB-Regulated Gene Expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell. Biochem. 2011, 354, 189–197. [Google Scholar] [CrossRef]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef]
- Avasthi, A.S.; Jawaid, S.A.; Jain, S.; Bhatnagar, M.; Purkayastha, S.; Ghosal, S. Free radical scavenging and antioxidant impact of Indian medicinal plants extracts on H2O2 mediated oxidative stress on human erythrocyte. Am. J. Phytomed. Clin. Ther. 2014, 2, 1052–1069. [Google Scholar]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef]
- Mathai, N.J.; Sony, D.; Mane, P.P.; Shetty, C.B.; Latheef, L.; Kamath, K.; Khaleed, M.; Kochikuzhyil, B.M.; Baliga, M.S. Antiarthritic Effects of Turmeric and Curcumin: A Revisit. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–252. [Google Scholar]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.-L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rizvi, S.I. Modulation Effects of Curcumin on Erythrocyte Ion-Transporter Activity. Int. J. Cell Biol. 2015, 2015, 630246. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Sinha, A.; Chu, T.T.T.; Dao, M.; Chandramohanadas, R. Single-cell evaluation of red blood cell bio-mechanical and nano-structural alterations upon chemically induced oxidative stress. Sci. Rep. 2015, 5, 9768. [Google Scholar] [CrossRef] [PubMed]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Reports 2020, 93. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Persiani, S.; Rotini, R.; Trisolino, G.; Rovati, L.C.; Locatelli, M.; Paganini, D.; Antonioli, D.; Roda, A. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthr. Cartil. 2007, 15, 764–772. [Google Scholar] [CrossRef]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2022, 12, 485–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.; Bigioni, I.; Misiti, F.; Fattorini, L.; Scotto d’Abusco, A.; Rodio, A. The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis. Curr. Issues Mol. Biol. 2022, 44, 3481-3495. https://doi.org/10.3390/cimb44080240
Mariano A, Bigioni I, Misiti F, Fattorini L, Scotto d’Abusco A, Rodio A. The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis. Current Issues in Molecular Biology. 2022; 44(8):3481-3495. https://doi.org/10.3390/cimb44080240
Chicago/Turabian StyleMariano, Alessia, Irene Bigioni, Francesco Misiti, Luigi Fattorini, Anna Scotto d’Abusco, and Angelo Rodio. 2022. "The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis" Current Issues in Molecular Biology 44, no. 8: 3481-3495. https://doi.org/10.3390/cimb44080240
APA StyleMariano, A., Bigioni, I., Misiti, F., Fattorini, L., Scotto d’Abusco, A., & Rodio, A. (2022). The Nutraceuticals as Modern Key to Achieve Erythrocyte Oxidative Stress Fighting in Osteoarthritis. Current Issues in Molecular Biology, 44(8), 3481-3495. https://doi.org/10.3390/cimb44080240






