The Pathological Links between Adiposity and the Carpal Tunnel Syndrome
Abstract
1. Introduction
2. General Considerations about the Pathophysiology of the Carpal Tunnel Syndrome
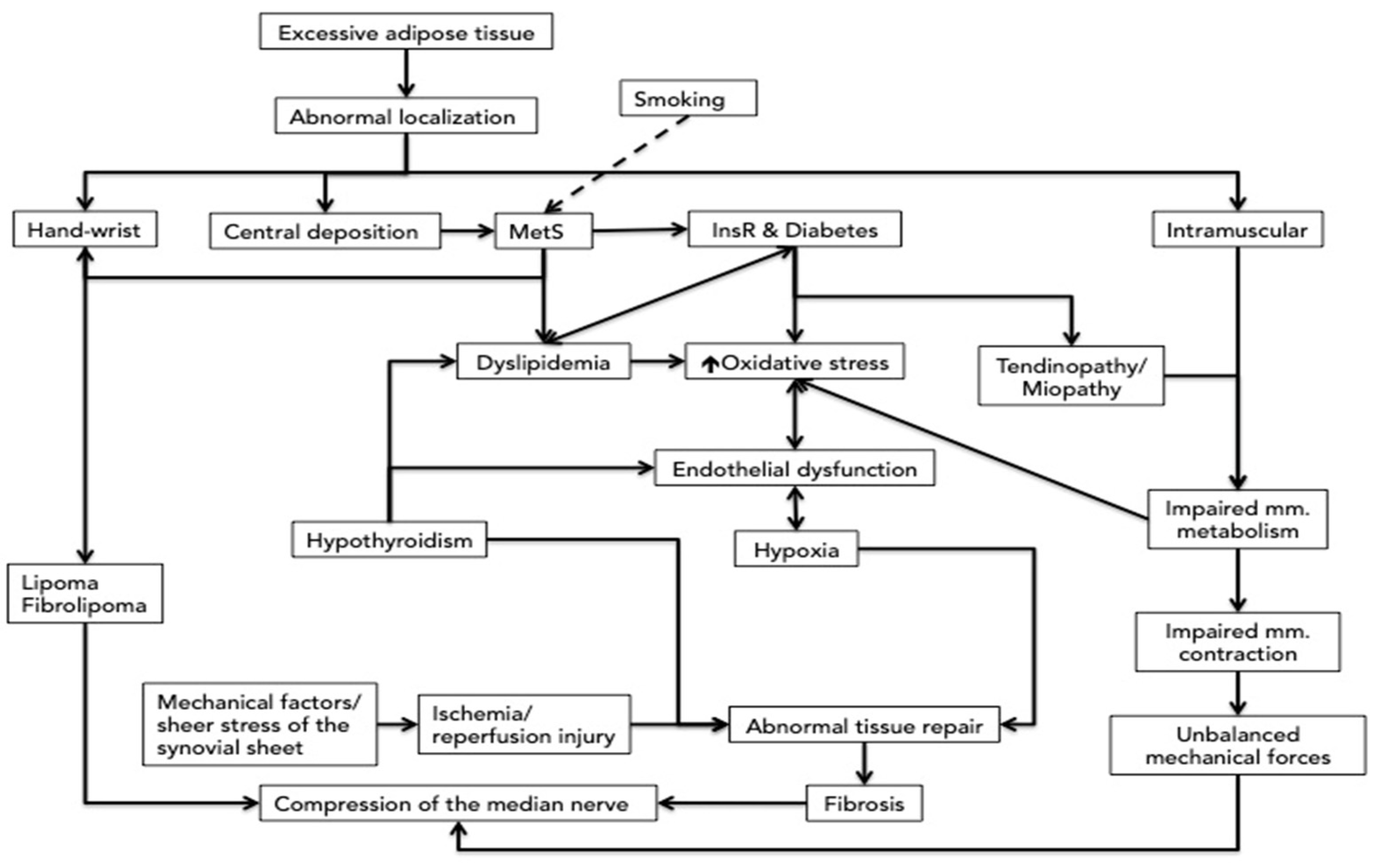
3. The Direct Compression and the Association with the Metabolic Syndrome of the Fat Deposition around the Wrist
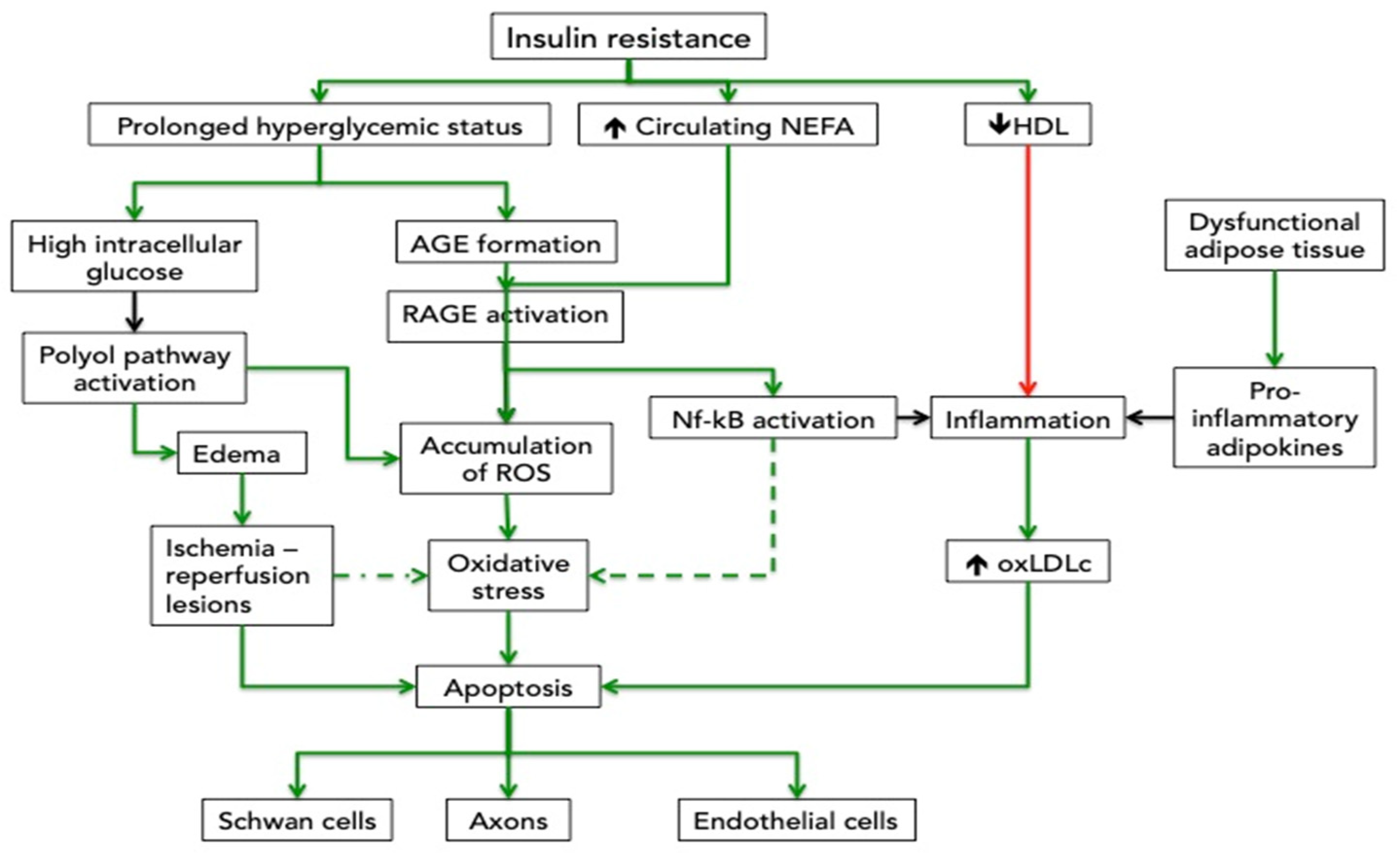
4. Insulin Resistance, Dyslipidemia, Inflammatory and Oxidative Mechanisms Related to the Central Deposition of the Fat
4.1. Effects of Hyperglycemia on Nerve Impairment
4.2. Effects of Dyslipidemia on Nerve Impairment
4.3. Vascular Effects
5. The Impaired Muscle Contraction and Metabolism Related to Myosteatosis
5.1. Effects on Muscles and Tendons
5.2. Myosteatosis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuppin, P.; Blotière, P.O.; Weill, A.; Ricordeau, P.; Allemand, H. Carpal tunnel syndrome surgery in France in 2008: Patients’ characteristics and management. Rev. Neurol. 2011, 167, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, J.; Goerl, K. Carpal tunnel syndrome: Diagnosis and management. Am. Fam. Physician 2016, 94, 993–999. [Google Scholar] [PubMed]
- Burton, C.; Chesterton, L.; Davenport, G. Diagnosing and managing carpal tunnel syndrome in primary care. Br. J. Gen. Pr. 2014, 64, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Aroori, S.; Spence, R.A. Carpal tunnel syndrome. Ulster Med J. 2008, 77, 6–17. [Google Scholar]
- Loher, H.; Kreis, R.; Boesch, C.H.; Christ, E. The Flexibility of Ectopic Lipids. Int. J. Mol. Sci. 2016, 17, 1554. [Google Scholar] [CrossRef]
- Michelsen, H.; A Posner, M. Medical history of carpal tunnel syndrome. Hand Clin. 2002, 18, 257–268. [Google Scholar] [CrossRef]
- Chammas, M.; Boretto, J.; Burmann, L.M.; Ramos, R.M.; Dos Santos Neto, F.C.; Silva, J.B. Carpal tunnel syndrome—Part I (anatomy, physiology, etiology and diagnosis). Rev. Bras. Ortop. 2014, 49, 429–436. [Google Scholar] [CrossRef]
- Pacek, C.A.; Tang, J.; Goitz, R.J.; Kaufmann, R.A.; Li, Z.-M. Morphological Analysis of the Carpal Tunnel. HAND 2009, 5, 77–81. [Google Scholar] [CrossRef]
- Bower, J.A.; Stanisz, G.; Keir, P. An MRI evaluation of carpal tunnel dimensions in healthy wrists: Implications for carpal tunnel syndrome. Clin. Biomech. 2006, 21, 816–825. [Google Scholar] [CrossRef]
- Aboonq, M.S. Pathophysiology of carpal tunnel syndrome. Neurosciences 2015, 20, 4–9. [Google Scholar]
- Barr, A.E.; Barbe, M.F. Pathophysiological Tissue Changes Associated With Repetitive Movement: A Review of the Evidence. Phys. Ther. 2002, 82, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Molinari, W.J., 3rd; Elfar, J.C. The double crush syndrome. J. Hand Surg. Am. 2013, 38, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Coppieters, M.W. The double crush syndrome revisited—A Delphi study to reveal current expert views on mechanisms underlying dual nerve disorders. Man. Ther. 2011, 16, 557–562. [Google Scholar] [CrossRef]
- Osterman, A.L. The Double Crush Syndrome. Orthop. Clin. N. Am. 1988, 19, 147–155. [Google Scholar] [CrossRef]
- Moradi, A.; Sadr, A.; Ebrahimzadeh, M.H.; Hassankhani, G.G.; Mehrad-Majd, H. Does diabetes mellitus change the carpal tunnel release outcomes? Evidence from a systematic review and meta-analysis. J. Hand Ther. 2020, 33, 394–401. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Meister, B.M.; Hong, S.-G.; Shin, J.; Rath, M.; Sayoc, J.; Park, J.-Y. Healthy versus Unhealthy Adipose Tissue Expansion: The Role of Exercise. J. Obes. Metab. Syndr. 2022, 31, 37–50. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 921–9255. [Google Scholar] [CrossRef]
- Boyko, E.J.; Fujimoto, W.Y.; Leonetti, D.L.; Newell-Morris, L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care 2000, 23, 465–471. [Google Scholar] [CrossRef]
- Korac, A.; Srdic-Galic, B.; Stancic, A.; Otasevic, V.; Korac, B.; Jankovic, A. Adipokine signatures of subcutaneous and visceral abdominal fat in normal-weight and obese women with different metabolic profiles. Arch. Med Sci. 2021, 17, 323–336. [Google Scholar] [CrossRef] [PubMed]
- McTernan, P.G.; McTernan, C.L.; Chetty, R.; Jenner, K.; Fisher, F.M.; Lauer, M.N.; Crocker, J.; Barnett, A.H.; Kumar, S. Increased resistin gene and protein expression in human abdominal adipose tissue. J. Clin. Endocrinol. Metab. 2002, 87, 2407. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Bravo, S.B.; Pérez-Sotelo, D.; Alonso, J.; Castro, A.I.; Baamonde, I.; Baltar, J.; Casanueva, F.F.; Pardo, M. CILAIR-Based Secretome Analysis of Obese Visceral and Subcutaneous Adipose Tissues Reveals Distinctive ECM Remodeling and Inflammation Mediators. Sci. Rep. 2015, 5, 12214. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Goodpaster, B.H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 2012, 23, 391–398. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [CrossRef]
- Addison, O.; Marcus, R.L.; LaStayo, P.C.; Ryan, A.S. Intermuscular Fat: A Review of the Consequences and Causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef]
- Yim, J.-E.; Heshka, S.; Albu, J.; Heymsfield, S.; Kuznia, P.; Harris, T.; Gallagher, D. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int. J. Obes. 2007, 31, 1400–1405. [Google Scholar] [CrossRef]
- Hafer-Macko, C.E.; Yu, S.; Ryan, A.S.; Ivey, F.M.; Macko, R.F. Elevated Tumor Necrosis Factor-α in Skeletal Muscle After Stroke. Stroke 2005, 36, 2021–2023. [Google Scholar] [CrossRef]
- Capizzi, M.; Leto, G.; Petrone, A.; Zampetti, S.; Papa, R.E.; Osimani, M.; Spoletini, M.; Lenzi, A.; Osborn, J.; Mastantuono, M.; et al. Wrist circumference is a clinical marker of insulin resistance in overweight and obese children and ad-olescents. Circulation 2011, 123, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Obirikorang, C.; Obirikorang, Y.; Acheampong, E.; Anto, E.O.; Toboh, E.; Asamoah, E.A.; Amakwaa, B.; Batu, E.N.; Brenya, P. Association of Wrist Circumference and Waist-to-Height Ratio with Cardiometabolic Risk Factors among Type II Diabetics in a Ghanaian Population. J. Diabetes Res. 2018, 2018, 1838162. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Pollock, N.K.; Casazza, K.; Clemens, T.L.; Goree, L.L.; Granger, W.M. Associations of Total and Undercarboxylated Osteocalcin With Peripheral and Hepatic Insulin Sensitivity and β-Cell Function in Overweight Adults. J. Clin. Endocrinol. Metab. 2013, 98, E1173–E1180. [Google Scholar] [CrossRef] [PubMed]
- Luordi, C.; Maddaloni, E.; Bizzarri, C.; Pedicelli, S.; Zampetti, S.; D’Onofrio, L.; Moretti, C.; Cappa, M.; Buzzetti, R. Wrist circumference is a biomarker of adipose tissue dysfunction and cardiovascular risk in children with obesity. J. Endocrinol. Investig. 2019, 43, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, A.; Soare, A.; Popa, S.G.; Tudor, M.N.; Moţa, M.; Pozzilli, P. Wrist Circumference: An Independent Predictor of Both Insulin Resistance and Chronic Kidney Disease in an Elderly Population. Rom. J. Diabetes Nutr. Metab. Dis. 2013, 20, 323–329. [Google Scholar] [CrossRef][Green Version]
- Ajithkumar, P.V.; Manju, L.; Deppa, M. Correlation of wrist circumference with waist circumference and body mass index in adults with early-onset type 2 diabetes mellitus. Int. J. Res. Med Sci. 2019, 7, 3322–3328. [Google Scholar] [CrossRef]
- Mousapour, P.; Barzin, M.; Valizadeh, M.; Mahdavi, M.; Hadaegh, F.; Azizi, F.; Hosseinpanah, F. Wrist circumference as a novel predictor of transition from metabolically healthy to unhealthy phenotype in overweight/obese adults: A gender-stratified 15.5-year follow-up. BMC Public Health 2021, 21, 2276. [Google Scholar] [CrossRef]
- Burt, A.M.; Huang, B.K. Imaging review of lipomatous musculoskeletal lesions. SICOT J. 2017, 3, 34. [Google Scholar] [CrossRef]
- Tahiri, Y.; Xu, L.; Kanevsky, J.; Luc, M. Lipofibromatous hamartoma of the median nerve: A comprehensive review and sys-tematic approach to evaluation, diagnosis, and treatment. J. Hand Surg. Am. 2013, 38, 2055–2067. [Google Scholar] [CrossRef]
- Marek, T.; Mahan, M.A.; Carter, J.M.; Hove, B.M.; Bartos, R.; Amrami, K.K.; Spinner, R.J. What’s known and what’s new in adipose lesions of peripheral nerves? Acta Neurochir. 2021, 163, 835–842. [Google Scholar] [CrossRef]
- Kossoko, H.; Allah, C.K.; Kadio, M.R.; Yéo, S.; Assi-Djè Bi Djè, V.; Gueu, M. Fibrolipoma of the median nerve. A case report. Chir. De La Main 2008, 27, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.P.; Carneiro, J.Z. Peripheral nerve compression secondary to adjacent lipomas. Surg. Neurol. 2007, 67, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.; Givissis, P.; Christodoulou, A. Complications arising from a misdiagnosed giant lipoma of the hand and palm: A case report. J. Med. Case Rep. 2011, 5, 552. [Google Scholar] [CrossRef] [PubMed]
- Nevill, A.; Duncan, M.; Myers, T. BMI is dead; long live waist-circumference indices: But which index should we choose to predict cardio-metabolic risk? Nutr. Metab. Cardiovasc. Dis. 2022. [Google Scholar] [CrossRef]
- Mondelli, M.; Aretini, A.; Ginanneschi, F.; Greco, G.; Mattioli, S. Waist circumference and waist-to-hip ratio in carpal tunnel syndrome: A case–control study. J. Neurol. Sci. 2014, 338, 207–213. [Google Scholar] [CrossRef]
- Burton, C.L.; Chen, Y.; Chesterton, L.S.; A Van Der Windt, D. Trends in the prevalence, incidence and surgical management of carpal tunnel syndrome between 1993 and 2013: An observational analysis of UK primary care records. BMJ Open 2018, 8, e020166. [Google Scholar] [CrossRef]
- Sassi, S.A.; Giddins, G. Gender differences in carpal tunnel relative cross-sectional area: A possible causative factor in idiopathic carpal tunnel syndrome. J. Hand Surg. 2016, 41, 638–642. [Google Scholar] [CrossRef]
- Stachenfeld, N.S. Sex Hormone Effects on Body Fluid Regulation. Exerc. Sport Sci. Rev. 2008, 36, 152–159. [Google Scholar] [CrossRef]
- Ge, H.; Yang, Z.; Li, X.; Liu, D.; Li, Y.; Pan, Y.; Luo, D.; Wu, X. The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci. Rep. 2020, 10, 20034. [Google Scholar] [CrossRef]
- Mondelli, M.; Giannini, F.; Giacchi, M. Carpal tunnel syndrome incidence in a general population. Neurology 2002, 58, 289–294. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011-2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Hanewinckel, R.; Drenthen, J.; Ligthart, S.; Dehghan, A.; Franco, O.H.; Hofman, A.; Ikram, M.A.; Van Doorn, P.A. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: A prospective population-based cohort study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Singleton, J.R. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J. Neurol. Sci. 2006, 242, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ji, L.; Chang, J.; Wen, J.; Zhao, W.; Shi, H.; Zhou, L.; Li, Y.; Hu, R.; Hu, J.; et al. Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol. Metab. Syndr. 2015, 7, 14. [Google Scholar] [CrossRef]
- Rota, E.; Quadri, R.; Fanti, E.; Isoardo, G.; Poglio, F.; Tavella, A.; Paolasso, I.; Ciaramitaro, P.; Bergamasco, B.; Cocito, D. Electrophysiological findings of peripheral neuropathy in newly diagnosed type II diabetes mellitus. J. Peripher. Nerv. Syst. 2005, 10, 348–353. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J. Diabetes Investig. 2011, 2, 170–175. [Google Scholar] [CrossRef]
- Mantovani, A.; Rigolon, R.; Mingolla, L.; Pichiri, I.; Cavalieri, V.; Salvotelli, L.; Stoico, V.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J. Diabetes its Complicat. 2017, 31, 1021–1026. [Google Scholar] [CrossRef]
- Yan, L.; Mu, B.; Guan, Y.; Liu, X.; Zhao, N.; Pan, D.; Wang, S. Assessment of the relationship between non-alcoholic fatty liver disease and diabetic complications. J. Diabetes Investig. 2016, 7, 889–894. [Google Scholar] [CrossRef]
- Huang, J.; Li, R.; Liu, N.; Yi, N.; Zheng, H.; Zhang, Q.; Zhou, L.; Zhou, L.; Hu, R.; Lu, B. Liver fibrosis is independently associated with diabetic peripheral neuropathy in type 2 diabetes mellitus. J. Diabetes Investig. 2021, 12, 2019–2027. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Jung, C.-H.; Mok, J.-O.; Kang, S.K.; Kim, C.-H. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J. Diabetes Investig. 2013, 5, 170–175. [Google Scholar] [CrossRef]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J. Diabetes Its Complicat. 2020, 34, 107684. [Google Scholar] [CrossRef] [PubMed]
- Misawa, S.; Kuwabara, S.; Ogawara, K.; Kitano, Y.; Yagui, K.; Hattori, T. Hyperglycemia alters refractory periods in human diabetic neuropathy. Clin. Neurophysiol. 2004, 115, 2525–2529. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.K.; Orrick, J.A. Biochemistry, Polyol or Sorbitol Pathways; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576381/ (accessed on 11 March 2022).
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef]
- Hao, W.; Tashiro, S.; Hasegawa, T.; Sato, Y.; Kobayashi, T.; Tando, T.; Katsuyama, E.; Fujie, A.; Watanabe, R.; Morita, M.; et al. Hyperglycemia Promotes Schwann Cell De-differentiation and De-myelination via Sorbitol Accumulation and Igf1 Protein Down-regulation. J. Biol. Chem. 2015, 290, 17106–17115. [Google Scholar] [CrossRef]
- Kassem, H.S.; Azar, S.T.; Zantout, M.S.; Sawaya, R.A. Hypertriglyceridemia and peripheral neuropathy in neurologically asymptomatic patients. Neuroendocrinol. Lett. 2005, 26, 775–779. [Google Scholar]
- Wiggin, T.D.; Sullivan, K.A.; Pop-Busui, R.; Amato, A.; Sima, A.A.; Feldman, E.L. Elevated Triglycerides Correlate With Progression of Diabetic Neuropathy. Diabetes 2009, 58, 1634–1640. [Google Scholar] [CrossRef]
- Beyca, H.; Mesci, B.; Caklili, O.T.; Mutlu, H.; Oguz, A. Neuropathy Associated with Hypertriglyceridemia in Patients with Metabolic Syndrome. Acta Endocrinol. 2016, 12, 26–29. [Google Scholar] [CrossRef]
- Kwai, N.C.G.; Nigole, W.; Poynten, A.M.; Brown, C.; Krishnan, A.V. The Relationship between Dyslipidemia and Acute Axonal Function in Type 2 Diabetes Mellitus In Vivo. PLoS ONE 2016, 11, e0153389. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, Y.; Zhang, J. A systematic review and meta-analysis of the serum lipid profile in prediction of diabetic neuropathy. Sci. Rep. 2021, 11, 499. [Google Scholar] [CrossRef]
- Duran, E.K.; Aday, A.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Hasanova, N.; Julius, U.; Obrosova, I.G. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: Role for oxidative–nitrosative stress. Free Radic. Biol. Med. 2012, 52, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Increased permeability across the blood-nerve barrier of albumin glycated in vitro and in vivo from patients with diabetic polyneuropathy. Proc. Natl. Acad. Sci. USA 1992, 89, 2218–2222. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Profirovic, J.; Pan, H.; Vaiskunaite, R.; Voyno-Yasenetskaya, T. G protein βγ subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: Regulation of cell shape and reactive oxygen species production. Circ. Res. 2003, 93, 848–856. [Google Scholar] [CrossRef]
- Holvoet, P.; Lee, D.-H.; Steffes, M.; Gross, M.; Jacobs, D.R., Jr. Association Between Circulating Oxidized Low-Density Lipoprotein and Incidence of the Metabolic Syndrome. Obstet. Gynecol. Surv. 2008, 63, 575–576. [Google Scholar] [CrossRef][Green Version]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-Induced Neuropathy in Mice. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef]
- Jang, E.-R.; Lee, C.S. 7-Ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-κB and Akt pathways. Neurochem. Int. 2011, 58, 52–59. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Gavini, C.K.; Bonomo, R.; Mansuy-Aubert, V. Neuronal LXR Regulates Neuregulin 1 Expression and Sciatic Nerve-Associated Cell Signaling in Western Diet-fed Rodents. Sci. Rep. 2020, 10, 6396. [Google Scholar] [CrossRef]
- Gavini, C.K.; Elshareif, N.; Aubert, G.; Germanwala, A.V.; Calcutt, N.A.; Mansuy-Aubert, V. LXR agonist improves peripheral neuropathy and modifies PNS immune cells in aged mice. J. Neuroinflamm. 2022, 19, 57. [Google Scholar] [CrossRef]
- Shiri, R. Hypothyroidism and carpal tunnel syndrome: A meta-analysis. Muscle Nerve 2014, 50, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Aldaghri, F.; Algahtani, M.S.; Almutairi, T.A.; Albusair, M.; Bin Ghali, K.; Al Asim, F.S. Prevalence of Hypothyroidism Among Carpal Tunnel Syndrome Patients at a Hospital in Saudi Arabia. Cureus 2020, 12, e12264. [Google Scholar] [CrossRef] [PubMed]
- Karne, S.S. Carpal Tunnel Syndrome in Hypothyroidism. J. Clin. Diagn. Res. 2016, 10, OC36–OC38. [Google Scholar] [CrossRef] [PubMed]
- Ruhla, S.; Weickert, M.O.; Arafat, A.; Osterhoff, M.; Isken, F.; Spranger, J.; Schöfl, C.; Pfeiffer, A.F.H.; Möhlig, M. A high normal TSH is associated with the metabolic syndrome. Clin. Endocrinol. 2010, 72, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.; Brenta, G. A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism. Front. Endocrinol. 2018, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Oge, A.; Sozmen, E.; Karaoglu, A.O. Effect of Thyroid Function on LDL Oxidation in Hypothyroidism and Hyperthyroidism. Endocr. Res. 2004, 30, 481–489. [Google Scholar] [CrossRef]
- Prieur, X.; Huby, T.; Coste, H.; Schaap, F.; Chapman, M.J.; Rodríguez, J.C. Thyroid Hormone Regulates the Hypotriglyceridemic Gene APOA5. J. Biol. Chem. 2005, 280, 27533–27543. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Gnoni, A.; Siculella, L. Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms. Int. J. Mol. Sci. 2017, 18, 744. [Google Scholar] [CrossRef]
- Gagnon, A.; Antunes, T.T.; Ly, T.; Pongsuwan, P.; Gavin, C.; Lochnan, H.A.; Sorisky, A. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism 2010, 59, 547–553. [Google Scholar] [CrossRef]
- Boone, L.R.; Lagor, W.; Moya, M.D.L.L.; Niesen, M.I.; Rothblat, G.H.; Ness, G.C. Thyroid hormone enhances the ability of serum to accept cellular cholesterol via the ABCA1 transporter. Atherosclerosis 2011, 218, 77–82. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Peng, D. Update on dyslipidemia in hypothyroidism: The mechanism of dyslipidemia in hypothyroidism. Endocr. Connect. 2022, 11, e210002. [Google Scholar] [CrossRef] [PubMed]
- Holovacova, D.; Kužma, M.; Killinger, Z.; Payer, J. Cross-sectional area of the median nerve is increased in primary autoimmune hypothyroidism and decreases upon treatment with thyroxine. Eur. J. Endocrinol. 2016, 175, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, L.; Mozzo, P.; Brugnone, F.; Zedde, A. Physiologicomathematical model for studying human exposure to organic solvents: Kinetics of blood/tissue n-hexane concentrations and of 2,5-hexanedione in urine. Occup. Environ. Med. 1986, 43, 760–768. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T. Toxic neuropathies: Mechanistic insights based on a chemical perspective. Neurosci. Lett. 2014, 596, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Otelea, M.; Handra, C.; Rascu, A. Registered cases of occupational n-hexane intoxication in Bucharest. Rom. J. Leg. Med. 2015, 23, 279–284. [Google Scholar] [CrossRef]
- Cohen, B.H.; Gaspar, M.; Daniels, A.H.; Akelman, E.; Kane, P.M. Multifocal Neuropathy: Expanding the Scope of Double Crush Syndrome. J. Hand Surg. 2016, 41, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Jin, R.C. Vascular nitric oxide: Formation and function. J. Blood Med. 2010, 1, 147–162. [Google Scholar] [CrossRef]
- Scholz, G.H.; Hanefeld, M. Metabolic Vascular Syndrome: New Insights into a Multidimensional Network of Risk Factors and Diseases. Visc. Med. 2016, 32, 319–326. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Contreras, C.; Sánchez, A.; Prieto, D. Role of Phosphatidylinositol 3-Kinase (PI3K), Mitogen-Activated Protein Kinase (MAPK), and Protein Kinase C (PKC) in Calcium Signaling Pathways Linked to the α1-Adrenoceptor in Resistance Arteries. Front. Physiol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Hirata, H.; Tsujii, M.; Yoshida, T.; Yoshida, K.I.; Morita, A.; Okuyama, N.; Nagakura, T.; Sugimoto, T.; Fujisawa, K.; Uchida, A. MMP-2 expression is associated with rapidly proliferative arteriosclerosis in the flexor tenosynovium and pain severity in carpal tunnel syndrome. J. Pathol. 2005, 205, 443–450. [Google Scholar] [CrossRef]
- Nagareddy, P.; Rajput, P.; Vasudevan, H.; McClure, B.; Kumar, U.; MacLeod, K.; McNeill, J. Inhibition of matrix metalloproteinase-2 improves endothelial function and prevents hypertension in insulin-resistant rats. J. Cereb. Blood Flow Metab. 2011, 165, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Stancu, C.S.; Botez, G.M.; Sima, A.V.; Simionescu, M. Irreversibly glycated LDL induce oxidative and inflammatory state in human endothelial cells; added effect of high glucose. Biochem. Biophys. Res. Commun. 2009, 390, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, L. The DDAH/ADMA pathway is a critical regulator of NO signalling in vascular homeostasis. Cell Adhes. Migr. 2008, 2, 149–150. [Google Scholar] [CrossRef][Green Version]
- Palomo, I.; Contreras, A.; Alarcon, M.; Leiva, E.; Guzman, L.; Mujica, V.; Icaza, G.; Díaz, N.; Gonzalez, D.; Moore-Carrasco, R. Elevated concentration of asymmetric dimethylarginine (ADMA) in individuals with metabolic syndrome. Nitric. Oxide 2011, 24, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Gluvic, Z.; Obradovic, M.M.; Sudar-Milovanovic, E.M.; Zafirovic, S.S.; Radak, D.J.; Essack, M.M.; Bajic, V.B.; Takashi, G.; Isenovic, E.R. Regulation of nitric oxide production in hypothyroidism. Biomed. Pharmacother. 2020, 124, 109881. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.; Ye, X.; Blakely, B.; Min, J.; Kong, W.; Zhang, N.; Gou, L.; Regmi, A.; Hu, S.Q.; et al. Association between the expression of vascular endothelial growth factors and metabolic syndrome or its components: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 62. [Google Scholar] [CrossRef]
- Hoffmann, S.; Hofbauer, L.C.; Scharrenbach, V.; Wunderlich, A.; Hassan, I.; Lingelbach, S.; Zielke, A. Thyrotropin (TSH)-Induced Production of Vascular Endothelial Growth Factor in Thyroid Cancer Cells in Vitro: Evaluation of TSH Signal Transduction and of Angiogenesis-Stimulating Growth Factors. J. Clin. Endocrinol. Metab. 2004, 89, 6139–6145. [Google Scholar] [CrossRef]
- Deger, A.N.; Deger, H.; Taser, F. The role of neoangiogenesis and vascular endothelial growth factor in the development of carpal tunnel syndrome in patients with diabetes. Niger. J. Clin. Pr. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Donato, G.; Galasso, O.; Valentino, P.; Conforti, F.; Zuccalà, V.; Russo, E.; Maltese, L.; Perrotta, I.; Tripepi, S.; Amorosi, A. Pathological findings in subsynovial connective tissue in idiopathic carpal tunnel syndrome. Clin. Neuropathol. 2009, 28, 129–135. [Google Scholar] [CrossRef]
- Stoian, A.; Bacârea, A.; Motataianu, A.; Stoian, M.; Gliga, F.; Bacârea, V.; Duicu, C.; Bănescu, C. Vascular Endothelial Growth Factor Insertion/Deletion gene polymorphism in patients with type 2 diabetes and diabetic peripheral polyneuropathy. Rev. Romana Med. Lab. 2014, 22, 165–172. [Google Scholar] [CrossRef][Green Version]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Lao, J.; Gu, Y.; Zhao, X.; Rui, J.; Gao, K. Case-control study on individual risk factors of carpal tunnel syndrome. Exp. Ther. Med. 2018, 15, 2761–2766. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, S.M.; Yoo, B.; Oh, B.; Kang, H.C. Associations of smoking with overall obesity, and central obesity: A cross-sectional study from the Korea National Health and Nutrition Examination Survey (2010–2013). Epidemiol. Health. 2016, 38, e2016020. [Google Scholar] [CrossRef] [PubMed]
- Graff-Iversen, S.; Hewitt, S.; Forsén, L.; Grøtvedt, L.; Ariansen, I. Associations of tobacco smoking with body mass distribution; a population-based study of 65,875 men and women in midlife. BMC Public Health 2019, 19, 1439. [Google Scholar] [CrossRef]
- Lv, J.; Chen, W.; Sun, D.; Li, S.; Millwood, I.Y.; Smith, M.; Guo, Y.; Bian, Z.; Yu, C.; Zhou, H.; et al. Gender-Specific Association between Tobacco Smoking and Central Obesity among 0.5 Million Chinese People: The China Kadoorie Biobank Study. PLoS ONE 2015, 10, e0124586. [Google Scholar] [CrossRef] [PubMed]
- Lanas, F.; Bazzano, L.; Rubinstein, A.; Calandrelli, M.; Chen, C.-S.; Elorriaga, N.; Gutierrez, L.; Manfredi, J.A.; Seron, P.; Mores, N.; et al. Prevalence, Distributions and Determinants of Obesity and Central Obesity in the Southern Cone of America. PLoS ONE 2016, 11, e0163727. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2021, 23, 120–133. [Google Scholar] [CrossRef]
- Chirila, M.; Ghita, I.; Handra, C.; Fulga, I. Genetic variants influencing smoking behavior and efficacy of smoking cessation therapies. Rom. Biotechnol. Lett. 2014, 19, 9727–9734. [Google Scholar]
- Wills, A.G.; Hopfer, C. Phenotypic and genetic relationship between BMI and cigarette smoking in a sample of UK adults. Addict. Behav. 2018, 89, 98–103. [Google Scholar] [CrossRef]
- Tuovinen, E.-L.; Saarni, S.E.; Männistö, S.; Borodulin, K.; Patja, K.; Kinnunen, T.H.; Kaprio, J.; Korhonen, T. Smoking status and abdominal obesity among normal- and overweight/obese adults: Population-based FINRISK study. Prev. Med. Rep. 2016, 4, 324–330. [Google Scholar] [CrossRef]
- Hulkkonen, S.; Shiri, R.; Auvinen, J.; Miettunen, J.; Karppinen, J.; Ryhänen, J. Risk factors of hospitalization for carpal tunnel syndrome among the general working population. Scand. J. Work. Environ. Health 2019, 46, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mihaltan, F.D.; Rajnoveanu, A.-G.; Rajnoveanu, R.-M. Impact of Smoking on Women During the Covid-19 Pandemic. Front. Med. 2021, 8, 584061. [Google Scholar] [CrossRef] [PubMed]
- Esen, A.M.; Barutcu, I.; Acar, M.; Degirmenci, B.; Kaya, D.; Turkmen, M.; Melek, M.; Onrat, E.; Esen, O.B.; Kirma, C. Effect of Smoking on Endothelial Function and Wall Thickness of Brachial Artery. Circ. J. 2004, 68, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Song, P.; Zou, M.-H. Obesity Paradox and Smoking Gun. Circ. Res. 2018, 122, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-L.; Zang, W.-J.; Lu, J.; Yu, X.-J.; Lin, Y.-X.; Cao, Y.-X. The Protective Effect of Captopril on Nicotine-Induced Endothelial Dysfunction in Rat. Basic Clin. Pharmacol. Toxicol. 2006, 99, 237–245. [Google Scholar] [CrossRef]
- Rinker, B.; Fink, B.F.; Barry, N.G.; Fife, J.A.; Milan, M.E.; Stoker, A.R.; Nelson, P.T. The effect of cigarette smoking on functional recovery following peripheral nerve ischemia/reperfusion injury. Microsurgery 2010, 31, 59–65. [Google Scholar] [CrossRef]
- Linardatou, V.; Karatzanos, E.; Panagopoulou, N.; Delis, D.; Kourek, C.; Rovina, N.; Nanas, S.; Vasileiadis, I. Passive smoking acutely affects the microcirculation in healthy non-smokers. Microvasc. Res. 2019, 128, 103932. [Google Scholar] [CrossRef]
- Henriksson, P.; Lu, Q.; Diczfalusy, U.; Freyschuss, A. Immediate Effect of Passive Smoking on Microcirculatory Flow. Microcirculation 2014, 21, 587–592. [Google Scholar] [CrossRef]
- Nakatani, T.; Nakashima, T.; Kita, T.; Ishihara, A. Effects of exposure to cigarette smoke at different dose levels on extensor digitorum longus muscle fibres in Wistar-Kyoto and spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 671–677. [Google Scholar] [CrossRef]
- Shiri, R.; Heliövaara, M.; Moilanen, L.; Viikari, J.; Liira, H.; Viikari-Juntura, E. Associations of cardiovascular risk factors, carotid intima-media thickness and manifest atherosclerotic vascular disease with carpal tunnel syndrome. BMC Musculoskelet. Disord. 2011, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Hegmann, K.T.; Thiese, M.S.; Kapellusch, J.; Merryweather, A.S.; Bao, S.; Silverstein, B.; Wood, E.M.; Kendall, R.; Wertsch, J.; Foster, J.; et al. Association Between Cardiovascular Risk Factors and Carpal Tunnel Syndrome in Pooled Occupational Cohorts. J. Occup. Environ. Med. 2016, 58, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chiang, J.-H.; Lay, I.-S.; Lee, Y.-C. Increased Risk of Coronary Artery Disease in People with a Previous Diagnosis of Carpal Tunnel Syndrome: A Nationwide Retrospective Population-Based Case-Control Study. BioMed Res. Int. 2019, 2019, 4206795. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, E.; Rørth, R.; Leicht, B.P.; Schou, M.; Maurer, M.S.; Kristensen, S.L.; Kober, L.; Gustafsson, F. Association of Carpal Tunnel Syndrome With Amyloidosis, Heart Failure, and Adverse Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2019, 74, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kaka, B.; Maharaj, S.S.; Fatoye, F. Prevalence of musculoskeletal disorders in patients with diabetes mellitus: A systematic review and meta-analysis. J. Back Musculoskelet. Rehabil. 2019, 32, 223–235. [Google Scholar] [CrossRef]
- Douloumpakas, I.; Pyrpasopoulou, A.; Triantafyllou, A.; Sampanis, C.; Aslanidis, S. Prevalence of musculoskeletal disorders in patients with type 2 diabetes mellitus: A pilot study. Hippokratia 2007, 11, 216–218. [Google Scholar]
- Singla, R.; Gupta, Y.; Kalra, S. Musculoskeletal effects of diabetes mellitus. J. Pak. Med Assoc. 2015, 65, 1024–1027. [Google Scholar]
- Meyer, P.; Lintingre, P.-F.; Pesquer, L.; Poussange, N.; Silvestre, A.; Dallaudière, B. The Median Nerve at the Carpal Tunnel and Elsewhere. J. Belg. Soc. Radiol. 2018, 102, 17. [Google Scholar] [CrossRef]
- Yoo, J.J.; Cho, N.H.; Lim, S.H.; Kim, H.A. Relationships Between Body Mass Index, Fat Mass, Muscle Mass, and Musculoskeletal Pain in Community Residents. Arthritis Rheumatol. 2014, 66, 3511–3520. [Google Scholar] [CrossRef]
- Ghasemi-Rad, M.; Nosair, E.; Vegh, A.; Mohammadi, A.; Akkad, A.; Lesha, E.; Mohammadi, M.H.; Sayed, D.; Davarian, A.; Maleki-Miyandoab, T.; et al. A handy review of carpal tunnel syndrome: From anatomy to diagnosis and treatment. World J. Radiol. 2014, 6, 284–300. [Google Scholar] [CrossRef]
- Dhananjaya, J.R.; Veena, H.C.; Mamatha, B.S.; Sudarshan, C.R. Comparative study of body mass index, hand grip strength, and handgrip endurance in healthy individuals. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 594–598. [Google Scholar]
- Pasdar, Y.; Darbandi, M.; Mirtaher, E.; Rezaeian, S.; Najafi, F.; Hamzeh, B. Associations between Muscle Strength with Dif-ferent Measures of Obesity and Lipid Profiles in Men and Women: Results from RaNCD Cohort Study. Clin. Nutr. Res. 2019, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Keevil, V.L.; Luben, R.; Dalzell, N.; Hayat, S.; Sayer, A.A.; Wareham, N.J.; Khaw, K.-T. Cross-sectional associations between different measures of obesity and muscle strength in men and women in a British cohort study. J. Nutr. Health Aging 2015, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Connizzo, B.K.; Bhatt, P.R.; Liechty, K.W.; Soslowsky, L.J. Diabetes Alters Mechanical Properties and Collagen Fiber Re-Alignment in Multiple Mouse Tendons. Ann. Biomed. Eng. 2014, 42, 1880–1888. [Google Scholar] [CrossRef]
- Studentsova, V.; Mora, K.M.; Glasner, M.F.; Buckley, M.R.; Loiselle, A.E. Obesity/Type II Diabetes Promotes Function-limiting Changes in Murine Tendons that are not reversed by Restoring Normal Metabolic Function. Sci. Rep. 2018, 8, 9218. [Google Scholar] [CrossRef]
- Grewal, N.; Thornton, G.M.; Behzad, H.; Sharma, A.; Lu, A.; Zhang, P.; Reid, W.D.; Granville, D.J.; Scott, D.J.G.A. Accumulation of Oxidized LDL in the Tendon Tissues of C57BL/6 or Apolipoprotein E Knock-Out Mice That Consume a High Fat Diet: Potential Impact on Tendon Health. PLoS ONE 2014, 9, e114214. [Google Scholar] [CrossRef]
- David, M.A.; Jones, K.H.; Inzana, J.A.; Zuscik, M.; Awad, H.A.; Mooney, R.A. Tendon Repair Is Compromised in a High Fat Diet-Induced Mouse Model of Obesity and Type 2 Diabetes. PLoS ONE 2014, 9, e91234. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; Macdonald, K.G.; Cunningham, P.R.G.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Berggren, J.R.; Boyle, K.; Chapman, W.H.; Houmard, J.A. Skeletal muscle lipid oxidation and obesity: Influence of weight loss and exercise. Am. J. Physiol. Metab. 2008, 294, E726–E732. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Jubeau, M.; Munzinger, U.; Bizzini, M.; Agosti, F.; De Col, A.; Lafortuna, C.L.; Sartorio, A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 101, 51–59. [Google Scholar] [CrossRef]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221, jeb163840. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Weedon, M.N.; Langenberg, C.; Jackson, A.U.; Lyssenko, V.; Sparsø, T.; Thorleifsson, G.; Grallert, H.; Ferrucci, L.; Maggio, M.; et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum. Mol. Genet. 2009, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Bonnieu, A.; Carnac, G.; Vernus, B. Myostatin in the Pathophysiology of Skeletal Muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar] [CrossRef]
- Kurose, S.; Onishi, K.; Takao, N.; Miyauchi, T.; Takahashi, K.; Kimura, Y. Association of serum adiponectin and myostatin levels with skeletal muscle in patients with obesity: A cross-sectional study. PLoS ONE 2021, 16, e0245678. [Google Scholar] [CrossRef]
- Cho, S.-A.; Joo, H.J.; Cho, J.-Y.; Lee, S.H.; Park, J.H.; Hong, S.J.; Yu, C.W.; Lim, D.-S. Visceral Fat Area and Serum Adiponectin Level Predict the Development of Metabolic Syndrome in a Community-Based Asymptomatic Population. PLoS ONE 2017, 12, e0169289. [Google Scholar] [CrossRef]
- Ingelsson, E.; Arnlöv, J.; Zethelius, B.; Vasan, R.S.; Flyvbjerg, A.; Frystyk, J.; Berne, C.; Hänni, A.; Lind, L.; Sundström, J. As-sociations of serum adiponectin with skeletal muscle morphology and insulin sensitivity. J. Clin. Endocrinol. Metab. 2009, 94, 953–957. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Can, B.; Kara, O.; Kizilarslanoglu, M.C.; Arik, G.; Aycicek, G.S.; Sumer, F.; Civelek, R.; Demirtas, C.; Ulger, Z. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 2016, 29, 745–752. [Google Scholar] [CrossRef]
- Huang, C.; Niu, K.; Momma, H.; Kobayashi, Y.; Guan, L.; Nagatomi, R. Inverse association between circulating adiponectin levels and skeletal muscle strength in Japanese men and women. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 42–49. [Google Scholar] [CrossRef]
- Krause, M.P.; Milne, K.J.; Hawke, T.J. Adiponectin—Consideration for its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals With Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association With Performance and Function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Houmard, J.A.; Pories, W.J.; Dohm, G.L. Is There a Metabolic Program in the Skeletal Muscle of Obese Individuals? J. Obes. 2011, 2011, 250496. [Google Scholar] [CrossRef]
- Tamilarasan, K.P.; Temmel, H.; Das, S.K.; Al Zoughbi, W.; Schauer, S.; Vesely, P.W.; Hoefler, G. Skeletal muscle damage and impaired regeneration due to LPL-mediated lipotoxicity. Cell Death Dis. 2012, 3, e354. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Theron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B.; et al. Skeletal Muscle Lipid Content and Oxidative Activity in Relation to Muscle Fiber Type in Aging and Metabolic Syndrome. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 566–576. [Google Scholar] [CrossRef]
- Choi, S.; Files, D.C.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. Ser. A 2015, 71, 557–564. [Google Scholar] [CrossRef]
- Sanna, M.; Franzin, C.; Pozzobon, M.; Favaretto, F.; Rossi, C.A.; Calcagno, A.; Scarda, A.; Prà, C.D.; Pilon, C.; Milan, G.; et al. Adipogenic potential of skeletal muscle satellite cells. Clin. Lipidol. 2009, 4, 245–265. [Google Scholar] [CrossRef]
- Aguiari, P.; Leo, S.; Zavan, B.; Vindigni, V.; Rimessi, A.; Bianchi, K.; Franzin, C.; Cortivo, R.; Rossato, M.; Vettor, R.; et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Walikonis, J.; Slawny, N.; Wei, Y.; Korsnes, S.; Doyle, J.C.; Wold, B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 2001, 189, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tosic, M.; Allen, A.; Willmann, D.; Lepper, C.; Kim, J.; Duteil, D.; Schüle, R. Lsd1 regulates skeletal muscle regeneration and directs the fate of satellite cells. Nat. Commun. 2018, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, Z.; Loughna, P.T. Adipogenic Differentiation of Muscle Derived Cells is Repressed by Inhibition of GSK-3 Activity. Front. Veter. Sci. 2018, 5, 110. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.-W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between sarcopenia level and metabolic syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.; Winwood, K.; Morse, C.; Onambélé, G.L. The impact of obesity on skeletal muscle architecture in untrained young vs. old women. J. Anat. 2014, 225, 675–684. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Gerber, C.; Schneeberger, A.G.; Hoppeler, H.; Meyer, D.C. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: A study in thirteen patients. J. Shoulder Elb. Surg. 2007, 16, 691–696. [Google Scholar] [CrossRef]
- Cobb, T.K.; An, K.-N.; Cooney, W.P.; Berger, R.A. Lumbrical Muscle Incursion into the Carpal Tunnel During Finger Flexion. J. Hand Surg. 1994, 19, 434–438. [Google Scholar] [CrossRef]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface 2015, 12, 20150365. [Google Scholar] [CrossRef]
- Pagano, A.F.; Brioche, T.; Arc-Chagnaud, C.; Demangel, R.; Chopard, A.; Py, G. Short-term disuse promotes fatty acid infil-tration into skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, T.; Rinnankoski-Tuikka, R.; Rintala, M.; Seppänen-Laakso, T.; Pöllänen, E.L.; Alen, M.; Sipilä, S.; Kaprio, J.; Kovanen, V.; Rahkila, P.; et al. Differences in Muscle and Adipose Tissue Gene Expression and Cardio-Metabolic Risk Factors in the Members of Physical Activity Discordant Twin Pairs. PLoS ONE 2010, 5, e12609. [Google Scholar] [CrossRef] [PubMed]
- Arentson-Lantz, E.J.; English, K.L.; Paddon-Jones, D.; Fry, C.S. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J. Appl. Physiol. 2016, 120, 965–975. [Google Scholar] [CrossRef]
- Manganotti, P.; Stella, A.B.; Ajcevic, M.; di Girolamo, F.G.; Biolo, G.; Franchi, M.V.; Monti, E.; Sirago, G.; Marusic, U.; Simunic, B.; et al. Peripheral nerve adaptations to 10 days of horizontal bed rest in healthy young adult males. Am. J. Physiol. Integr. Comp. Physiol. 2021, 321, R495–R503. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otelea, M.R.; Nartea, R.; Popescu, F.G.; Covaleov, A.; Mitoiu, B.I.; Nica, A.S. The Pathological Links between Adiposity and the Carpal Tunnel Syndrome. Curr. Issues Mol. Biol. 2022, 44, 2646-2663. https://doi.org/10.3390/cimb44060181
Otelea MR, Nartea R, Popescu FG, Covaleov A, Mitoiu BI, Nica AS. The Pathological Links between Adiposity and the Carpal Tunnel Syndrome. Current Issues in Molecular Biology. 2022; 44(6):2646-2663. https://doi.org/10.3390/cimb44060181
Chicago/Turabian StyleOtelea, Marina Ruxandra, Roxana Nartea, Florina Georgeta Popescu, Anatoli Covaleov, Brindusa Ilinca Mitoiu, and Adriana Sarah Nica. 2022. "The Pathological Links between Adiposity and the Carpal Tunnel Syndrome" Current Issues in Molecular Biology 44, no. 6: 2646-2663. https://doi.org/10.3390/cimb44060181
APA StyleOtelea, M. R., Nartea, R., Popescu, F. G., Covaleov, A., Mitoiu, B. I., & Nica, A. S. (2022). The Pathological Links between Adiposity and the Carpal Tunnel Syndrome. Current Issues in Molecular Biology, 44(6), 2646-2663. https://doi.org/10.3390/cimb44060181





