Deletion of Antigen-Presenting Cells in Lipopolysaccharide-Induced Acute Kidney Injury (AKI) Affects the Exacerbation and Repair in AKI
Abstract
1. Introduction
2. Materials and Methods
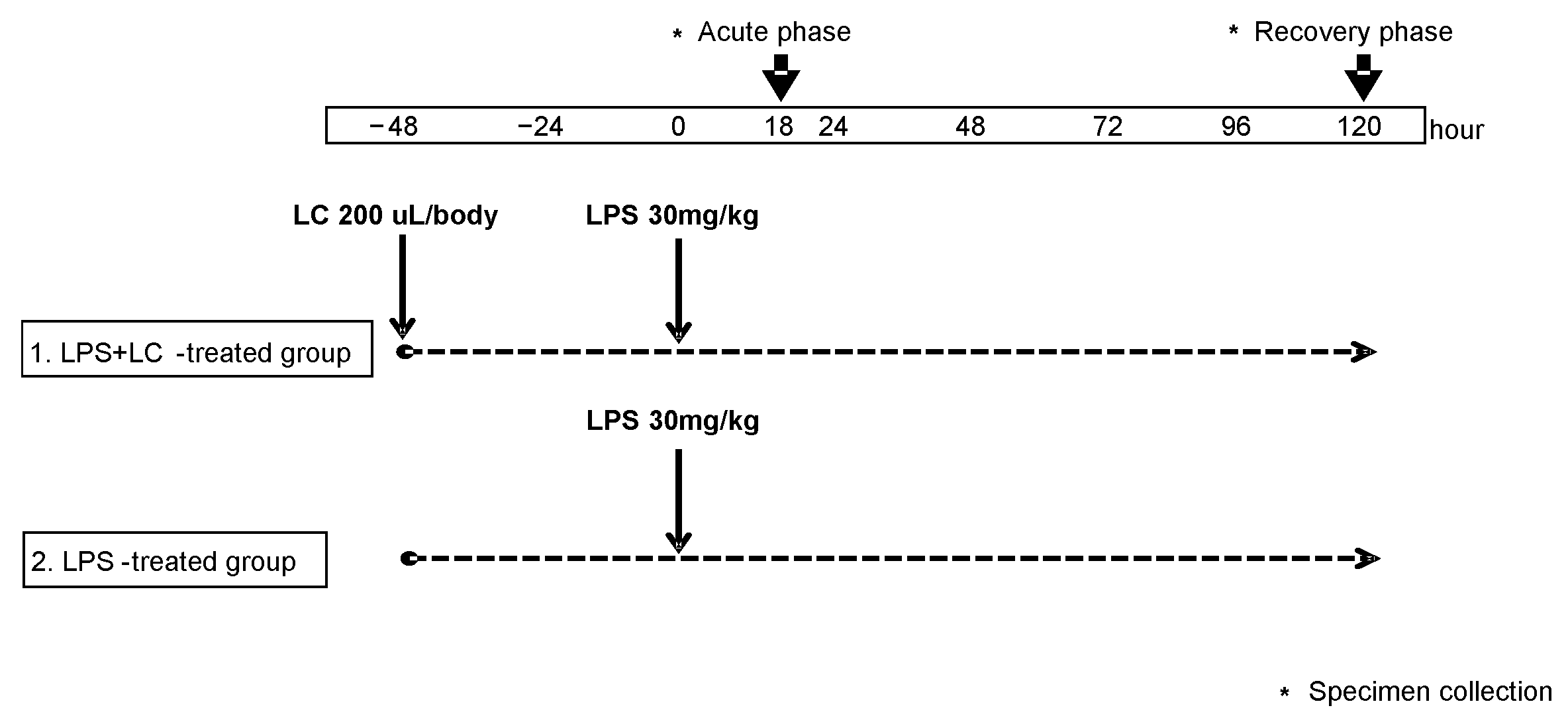
2.1. Experimental Protocol in the Murine Model of LPS-Induced AKI
2.2. Assessment of Kidney Immunohistochemistry
2.3. Assessment of Renal mRNA Expression
2.4. Cytokine Production
2.5. FACS Analysis
2.6. Statistical Analysis
3. Results
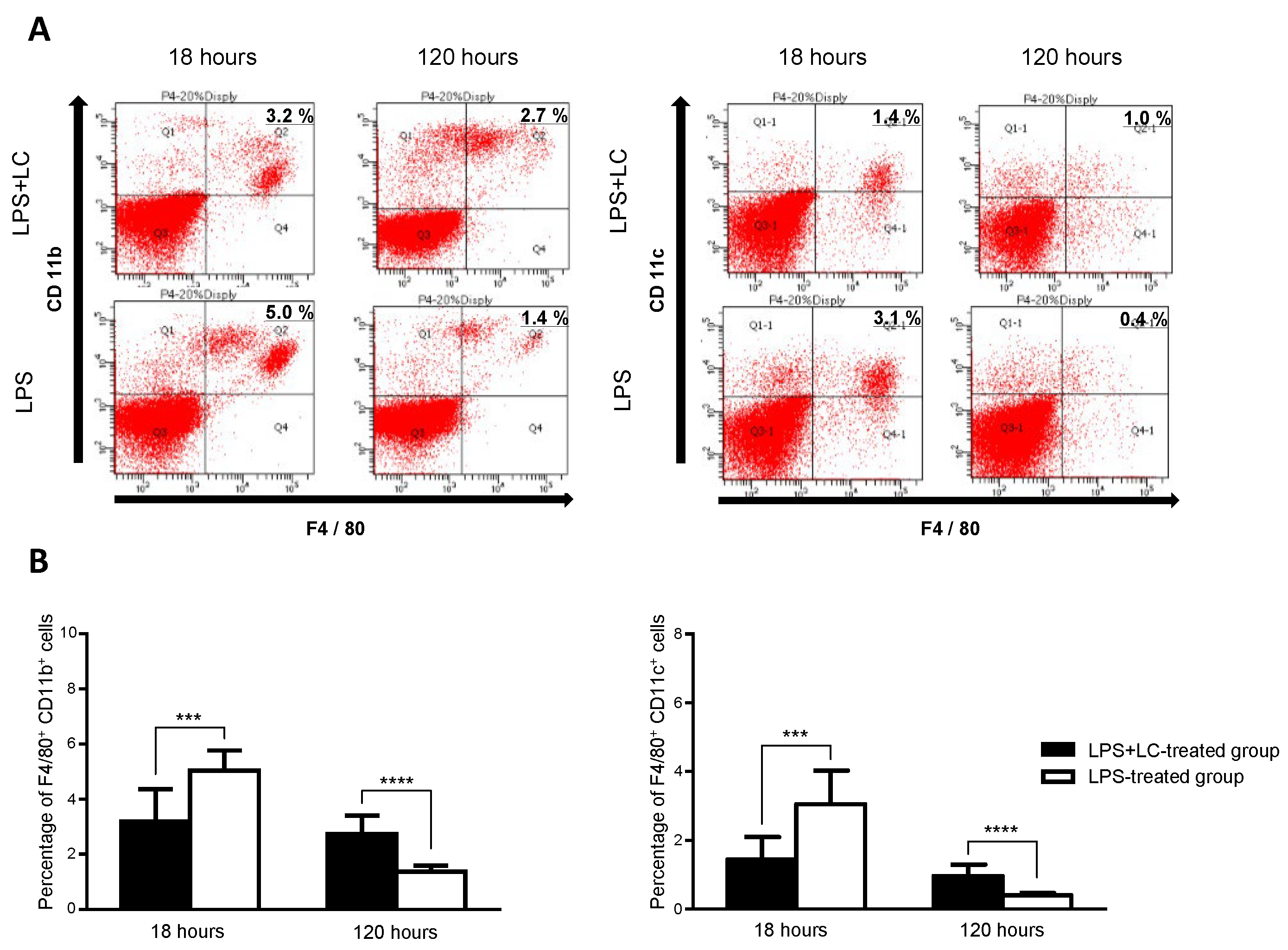
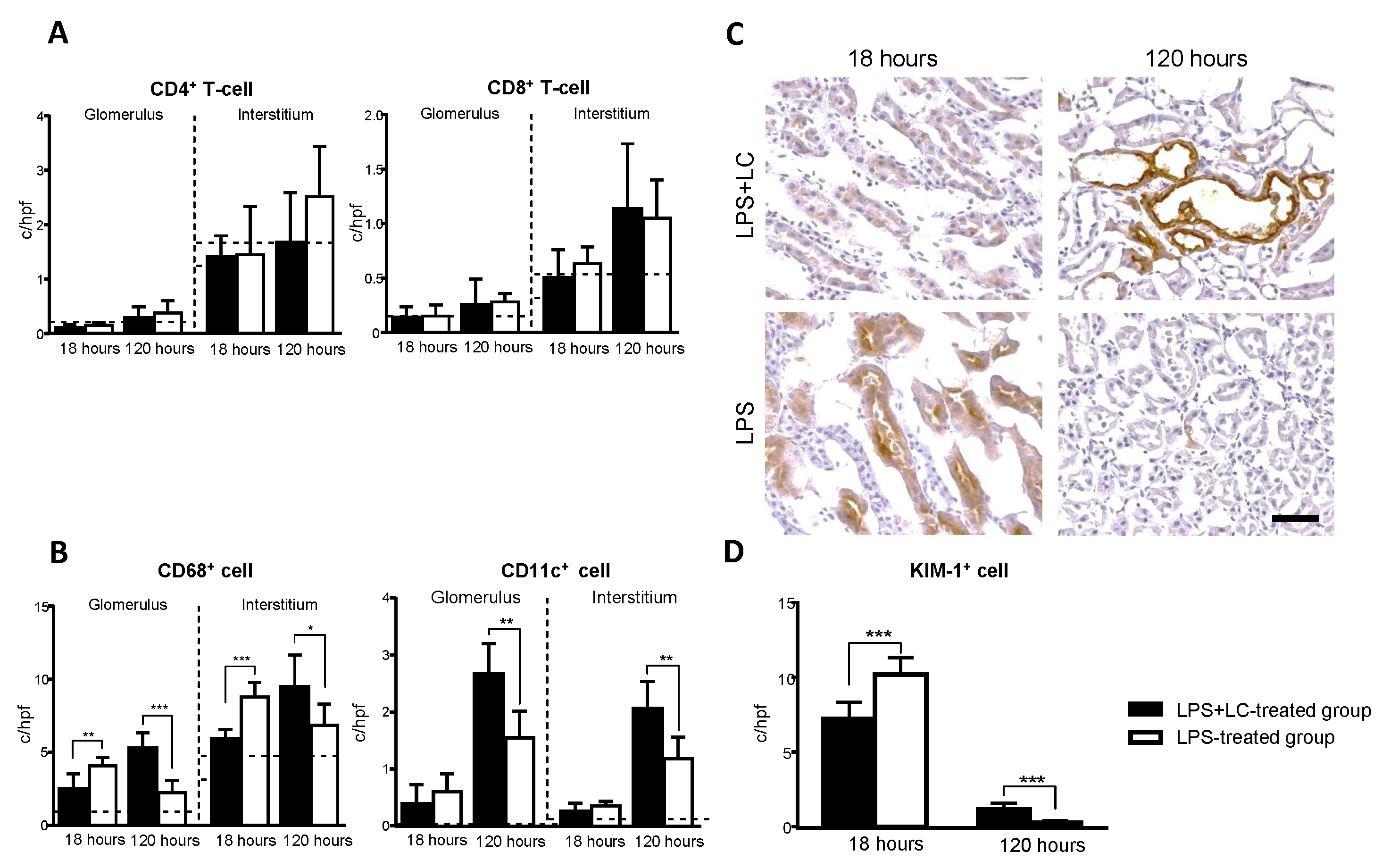
3.1. The LC Treatment Depleted Renal Macrophages and Dendritic Subset in the Mice
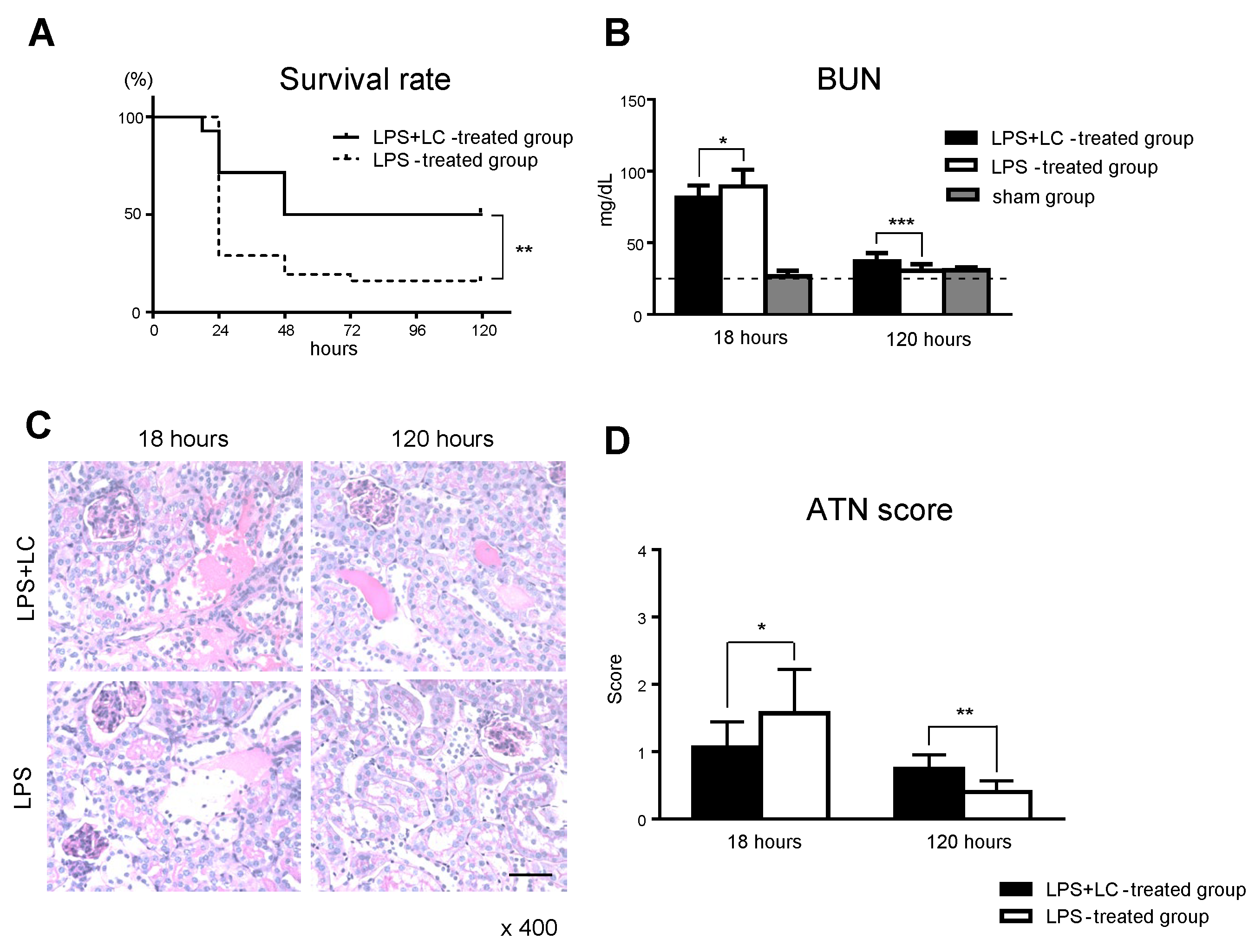
3.2. Macrophage and DC Depletion Improved the Survival Rate by Inhibiting the Renal Injury after LPS-Induced AKI
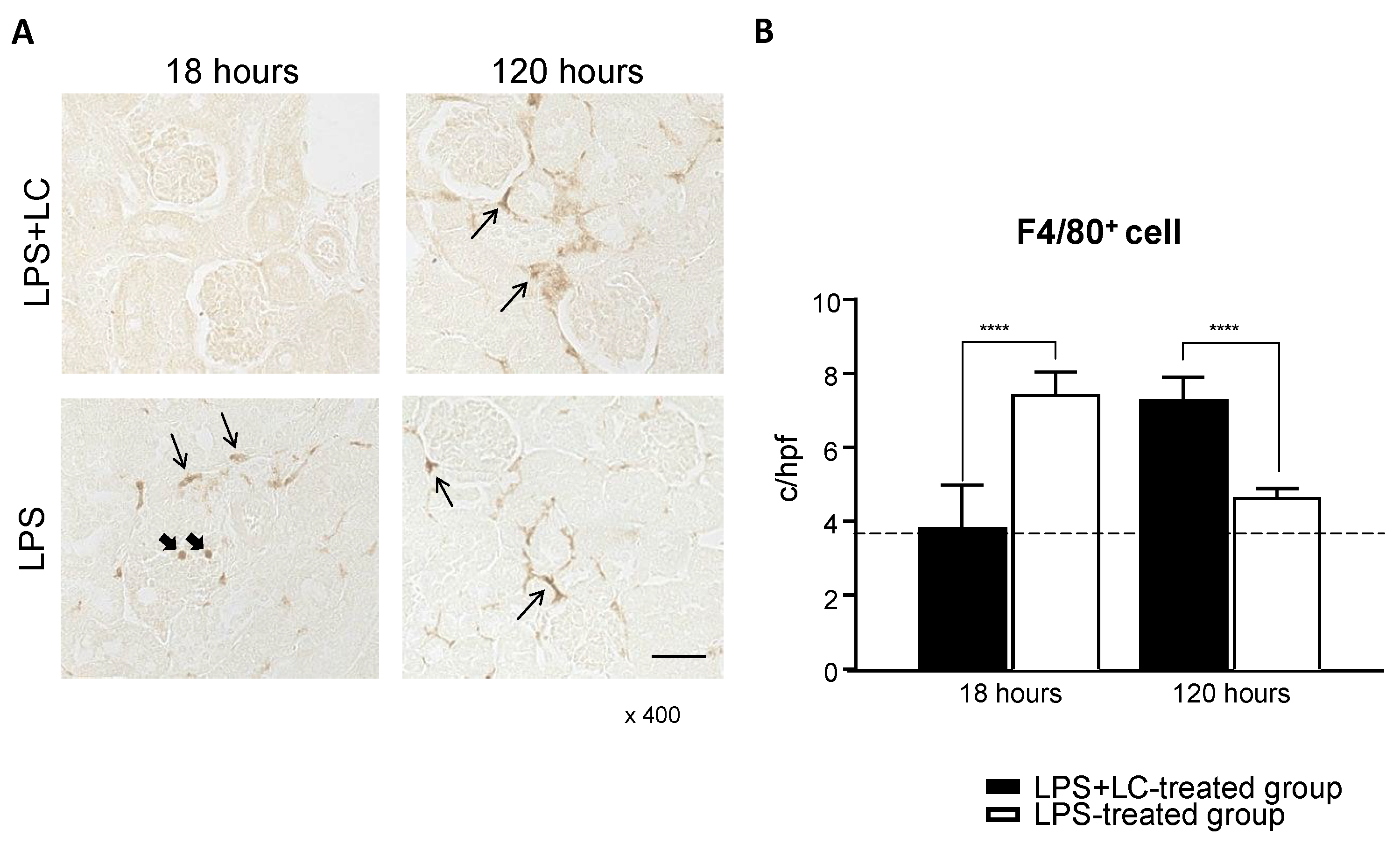
3.3. LC Treatment Affected Renal Injury, the Infiltration of Inflammatory Cells, and Tubular Damage in the Mouse Kidney after LPS Injection
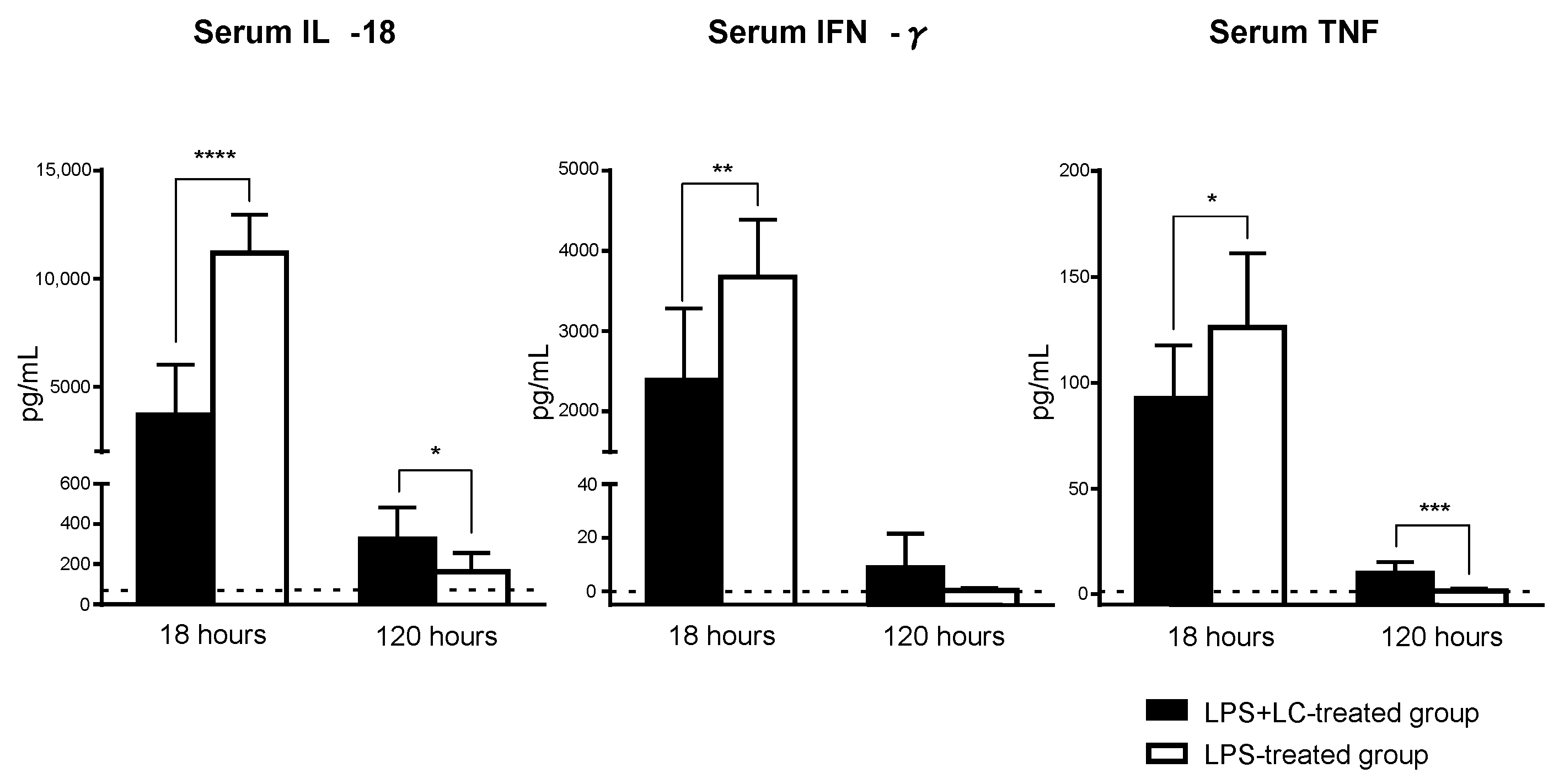
3.4. Serum Levels of Inflammatory Cytokines and the Intrarenal mRNA Expression of an Inflammatory Mediator after the LPS Injection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Zuk, A.; Bonventre, J.V. Acute kidney injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Takahashi, K.; Mizukami, H.; Kamata, K.; Inaba, W.; Kato, N.; Hibi, C.; Yagihashi, S. Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat. PLoS ONE 2012, 7, e30134. [Google Scholar] [CrossRef]
- Dong, X.; Swaminathan, S.; Bachman, L.A.; Croatt, A.J.; Nath, K.A.; Griffin, M.D. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005, 68, 1096–1108. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Chen, S.; Li, L.; Zhong, X.; Zhang, J.; Feng, Y.; Wang, L.; Chen, J.; Yu, M.; et al. Dimethyl fumarate attenuates LPS induced septic acute kidney injury by suppression of NFκB p65 phosphorylation and macrophage activation. Int. Immunopharmacol. 2022, 102, 108395. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.W.; Jung, M.H.; Cho, H.S.; Jeon, D.H.; Chang, S.H.; Park, D.J. Macrophage depletion ameliorates glycerol-induced acute kidney injury in mice. Nephron Exp. Nephrol. 2014, 128, 21–29. [Google Scholar] [CrossRef]
- Li, X.; Mu, G.; Song, C.; Zhou, L.; He, L.; Jin, Q.; Lu, Z. Role of M2 Macrophages in Sepsis-Induced Acute Kidney Injury. Shock 2018, 50, 233–239. [Google Scholar] [CrossRef]
- Rogers, N.M.; Ferenbach, D.A.; Isenberg, J.S.; Thomson, A.W.; Hughes, J. Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Funauchi, M.; Matsumura, I. Protective effects of recombinant human soluble thrombomodulin on lipopolysaccharide-induced acute kidney injury. Int. J. Mol. Sci. 2020, 21, 2519. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kinoshita, K.; Hino, S.; Yano, T.; Niki, K.; Hirooka, Y.; Kishimoto, K.; Funauchi, M.; Matsumura, I. Signaling Rho-kinase mediates inflammation and apoptosis in T cells and renal tubules in cisplatin nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2015, 308, F899–F909. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Yamagata, T.; Yoo, B.S.; Sugiyama, M.; Ikoma, S.; Kinoshita, K.; Funauchi, M.; Kanamaru, A. The beneficial effects of treatment with all-trans-retinoic acid plus corticosteroid on autoimmune nephritis in NZB/WF1 mice. Clin. Exp. Immunol. 2005, 139, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Bastos, K.R.; Barboza, R.; Sardinha, L.; Russo, M.; Alvarez, J.M.; Lima, M.R. Role of endogenous IFN-gamma in macrophage programming induced by IL-12 and IL-18. J. Interferon Cytokine Res. 2007, 27, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wyburn, K.; Wu, H.; Yin, J.; Jose, M.; Eris, J.; Chadban, S. Macrophage-derived interleukin-18 in experimental renal allograft rejection. Nephrol. Dial. Transplant. 2005, 20, 699–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alam, U. Immunity: The immune response to infectious and inflammatory disease. Yale J. Biol. Med. 2007, 80, 137. [Google Scholar]
- Ferenbach, D.A.; Sheldrake, T.A.; Dhaliwal, K.; Kipari, T.M.; Marson, L.P.; Kluth, D.C.; Hughes, J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012, 82, 928–933. [Google Scholar] [CrossRef]
- Kim, B.S.; Lim, S.W.; Li, C.; Kim, J.S.; Sun, B.K.; Ahn, K.O.; Han, S.W.; Kim, J.; Yang, C.W. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 2005, 79, 1370–1377. [Google Scholar] [CrossRef]
- Wu, C.J.; Sheu, J.R.; Chen, H.H.; Liao, H.F.; Yang, Y.C.; Yang, S.; Chen, Y.J. Modulation of monocyte-derived dendritic cell differentiation is associated with ischemic acute renal failure. J. Surg. Res. 2006, 132, 104–111. [Google Scholar] [CrossRef]
- Dong, X.; Swaminathan, S.; Bachman, L.A.; Croatt, A.J.; Nath, K.A.; Griffin, M.D. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007, 71, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Loi, P.; Paulart, F.; Pajak, B.; Nagy, N.; Salmon, I.; Moser, M.; Goldman, M.; Flamand, V. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant. Proc. 2004, 36, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| 18SrRNA | GTAACCCGTTGAACCCCATTC | GCCTCACTAAACCATCCAATCG |
| IFN-γ | TGCTGATGGGAGGAGATGTCT | TTTCTTTCAGGGACAGCCTGTT |
| TNF-α | CGATCACCCCGAAGTTCAGTA | GGTGCCTATGTCTCAGCCTCTT |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| CCL2/MCP-1 | AAAAACCTGGATCGGAACCAA | CGGGTCAACTTCACATTCAAAG |
| ICAM-1 | CATCCCAGAGAAGCCTTCCTG | TCAGCCACTGAGTCTCCAAGC |
| T-bet | CCTGGACCCAACTGTCAACT | AACTGTGTTCCCGAGGTGTC |
| GATA3 | AGGGACATCCTGCGCGAACTGT | CATCTTCCGGTTTCGGGTCTGG |
| Gene Name | TaqMan Gene Expression Assay ID |
|---|---|
| 18SrRNA | 4310893E |
| IL-6 | Mm00446190_m1 |
| IL-12p40 | Mm00434174_m1 |
| IL-18 | Mm00434226_m1 |
| KIM-1 | Mm00506686_m1 |
| 18 h | 120 h | |
|---|---|---|
| LPS + LC vs. LPS Treated Group | ||
| Cytokines | ||
| IFN-γ | 14.7 ± 1.6 vs. 23.6 ± 3.0 * | 4.0 ± 0.6 vs. 3.0 ± 0.5 |
| TNF | 24.9 ± 2.6 vs. 31.5 ± 1.2 * | 12.2 ± 3.3 vs. 8.0 ± 1.7 |
| IL-6 | 1314.0 ± 190.7 vs. 3900.0 ± 580.8 **** | 42.6 ± 9.8 vs. 17.4 ± 3.3 * |
| IL-10 | 56.2 ± 15.3 vs. 36.3 ± 11.1 | 5.8 ± 1.9 vs. 3.5 ± 0.5 |
| IL-12p40 | 0.6 ± 0.1 vs. 0.6 ± 0.1 | 0.9 ± 0.2 vs. 1.0 ± 0.1 |
| IL-18 | 5.9 ± 0.8 vs. 10.8 ± 0.9 ** | 1.8 ± 0.4 vs. 1.3 ± 0.1 |
| Chemokines | ||
| CCL2/MCP-1 | 75.0 ± 10.8 vs. 153.4 ± 18.5 ** | 13.4 ± 2.2 vs. 6.5 ± 1.5 * |
| KIM-1 | 89.8 ± 16.0 vs. 258.7 ± 45.1 ** | 26.6 ± 16.8 vs. 2.7 ± 1.5 ** |
| Th cell subset transcription factors | ||
| T-bet | 1.7 ± 0.3 vs. 3.5 ± 0.6 * | 3.7 ± 1.0 vs. 5.2 ± 0.6 |
| GATA3 | 0.9 ± 0.1 vs. 0.9 ± 0.1 | 0.9 ± 0.1 vs. 1.1 ± 0.1 |
| Leukocyte adhesion molecule | ||
| ICAM-1 | 61.3 ± 6.7 vs. 101.5 ± 16.7 * | 1.9 ± 0.3 vs. 1.9 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Nozaki, Y.; Akazawa, H.; Kishimoto, K.; Kinoshita, K.; Matsumura, I. Deletion of Antigen-Presenting Cells in Lipopolysaccharide-Induced Acute Kidney Injury (AKI) Affects the Exacerbation and Repair in AKI. Curr. Issues Mol. Biol. 2022, 44, 5655-5665. https://doi.org/10.3390/cimb44110383
Li J, Nozaki Y, Akazawa H, Kishimoto K, Kinoshita K, Matsumura I. Deletion of Antigen-Presenting Cells in Lipopolysaccharide-Induced Acute Kidney Injury (AKI) Affects the Exacerbation and Repair in AKI. Current Issues in Molecular Biology. 2022; 44(11):5655-5665. https://doi.org/10.3390/cimb44110383
Chicago/Turabian StyleLi, Jinhai, Yuji Nozaki, Hiroki Akazawa, Kazuya Kishimoto, Koji Kinoshita, and Itaru Matsumura. 2022. "Deletion of Antigen-Presenting Cells in Lipopolysaccharide-Induced Acute Kidney Injury (AKI) Affects the Exacerbation and Repair in AKI" Current Issues in Molecular Biology 44, no. 11: 5655-5665. https://doi.org/10.3390/cimb44110383
APA StyleLi, J., Nozaki, Y., Akazawa, H., Kishimoto, K., Kinoshita, K., & Matsumura, I. (2022). Deletion of Antigen-Presenting Cells in Lipopolysaccharide-Induced Acute Kidney Injury (AKI) Affects the Exacerbation and Repair in AKI. Current Issues in Molecular Biology, 44(11), 5655-5665. https://doi.org/10.3390/cimb44110383





