Abstract
In this study, 32 novel quinazolinone-scaffold-containing pyrazole carbamide derivatives were designed and synthesized in a search for a novel fungicide against Rhizoctonia solani. Single-crystal X-ray diffraction of 3-(difluoromethyl)-N-(2-((6,7-difluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a11) confirmed the structure of the target compounds. The in vitro antifungal activity of the target compounds against R. solani was evaluated at 100 µg/mL. The structure–activity relationship analysis results revealed that antifungal activity was highest when the substitution activity was at position 6. Moreover, the position and number of chlorine atoms directly affected the antifungal activity. Further in vitro bioassays revealed that 6a16 (EC50 = 9.06 mg/L) had excellent antifungal activity against R. solani that was higher than that of the commercial fungicide fluconazole (EC50 = 12.29 mg/L) but lower than that of bixafen (EC50 = 0.34 mg/L). Scanning electron microscopy), 7.33 (SEM) revealed that N-(2-((6,8-dichloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a16) also affected the mycelial morphology. The findings revealed that molecular hybridization was an effective tool for designing antifungal candidates. Meanwhile, pyrazolecarbamide derivatives bearing a quinazolinone fragment exhibited potential antifungal activity against R. solani.
1. Introduction
Rhizoctonia solani Kunh can infect the leaves and roots of various crops [1,2], causing significant yield loss. Specifically, R. solani causes sheath blight in rice, leading up to 40% yield loss [3,4]. Currently, chemical pesticides remain the most effective strategy for controlling rice sheath blight, because of the lack of rice sheath blight-resistant varieties. However, the abuse of chemical pesticides leads to pesticide resistance by pathogenic fungi. Therefore, new pesticide formulations that are more effective and more friendly to the environment need to be continuously developed.
Heterocyclic compounds are the largest class of organic compounds and play a significant role in the development of pesticides, irrespective of whether they are natural or synthetic [5,6]. New super-efficient pesticides, most of which contain heterocyclic compounds, are continuously being developed. Nitrogen-containing heterocyclic compounds, such as pyrazole and quinazolinone, have been found to have good anti-fungal activity.
Notably, pyrazole amides have become a hot spot in fungicide research because of their unique mechanism of action, safety, and efficiency [7]. Pesticide companies have developed pyrazole amide fungicides, such as isopyrazam [8], benzovindiflupyr [9], sedaxane [10], bixafen [11], and fluxapyroxad [12] (Figure 1), whose common feature is their connection by benzene rings as bridges.
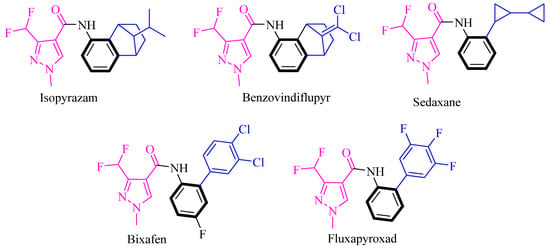
Figure 1.
Representative fungicides of pyrazolecarboxamides.
Quinazolinone is a benzopyrimidine heterocyclic compound that is the backbone structure of various alkaloids and drugs [13,14]. It has been widely used in the fields of medicine and pesticides because of its various excellent properties, such as its antifungal [15,16], antibacterial [17], antitumor [18], and antiviral activities [19,20]. For example, quinazolinone derivatives have been successfully developed into a commercial fungicide, fluconazole, which is used to control various fungal diseases caused by basidiomycetes, deuteromycetes, and discomycetes [21,22]. Albaconazole [23], which contains quinazolin-4-one, also exhibits a broad-spectrum antifungal activity (Figure 2).

Figure 2.
Representative fungicides of quinazolinone.
A molecular hybrid is a combination of two or more independently acting pharmacophores that are covalently linked [24]. It can be achieved by linking or by framework integration of two or more molecules to form one molecule with increased pharmacological activity. In this study, a quinazolinone structural unit and a pyrazole-containing active fragment were combined into one molecular structure. The two were connected using a benzene ring and used to design and synthesize a series of quinazolinone-containing pyrazole carboxamide derivatives (Figure 3). The molecular structure of the hybrid molecule was determined using 1H NMR, 13C NMR, and HRMS, followed by a preliminary screening of its antifungal activity in vitro. Subsequent physiological assays of compounds with high antifungal activity against R. solani, which causes sheath blight in rice, were further conducted.
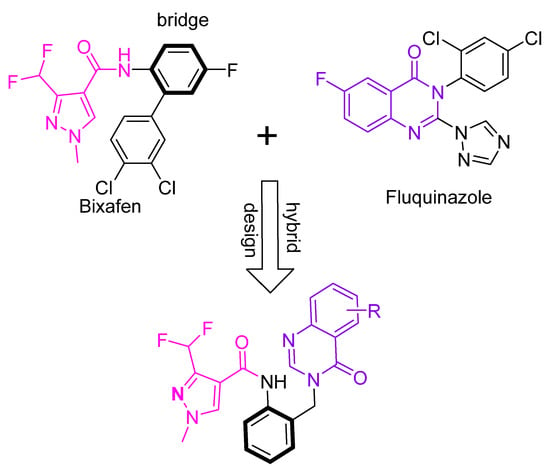
Figure 3.
The design strategy of the target compounds.
2. Materials and Methods
2.1. Chemistry
2.1.1. Instruments and Chemicals
1H and 13C NMR spectra were recorded in DMSO-d6 using 400 and 100 MHz spectrophotometers (Bruker BioSpin GmbH, Rheinstetten, Germany), respectively, while high-resolution mass spectrometry (HRMS) was performed using Thermo Scientific Q Exactive (Thermo Fisher Scientific, Waltham, MA, USA). The X-ray crystallographic data were collected and processed using a D8 Quest X-ray diffractometer (Bruker BioSpin GmbH, Rheinstetten, German). All solvents were distilled and dried using standard methods before use.
2.1.2. Synthesis of Intermediate 3
O-aminobenzyl alcohol (14.10 g, 100 mmol) was first added to 300 mL of saturated sodium bicarbonate solution, followed by dropwise addition of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbonyl chloride [25] (3, 23.28 g, 120 mmol) at room temperature. A huge amount of white solid was produced and filtered. The filtered cake was then recrystallized from ethanol to obtain a white product 3-(difluoromethyl)-N-(2-(hydroxymethyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (3, Scheme 1). Intermediate 3, white solid, yield 90%, m.p. 199.7–200.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.74 (s, 1H, NH), 8.39 (s, 1H, pyrazol-H5), 7.57 (d, J = 7.5 Hz, 1H, Ar-H5), 7.45 (dd, J = 7.6, 1.7 Hz, 1H, Ar-H2), 7.32 (t, J = 54.3 Hz, 1H, CF2H), 7.28 (td, J = 7.6, 1.7 Hz, 1H, Ar-H3), 7.20 (td, J = 7.5, 1.4 Hz, 1H, Ar-H4), 5.43 (s, 1H, OH), 4.56 (s, 2H, CH2), 3.97 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 159.79, 144.88 (t, J = 23.3 Hz), 135.36, 135.08, 132.68, 127.38, 127.11, 125.18, 124.44, 116.08 (t, J = 3.6 Hz), 109.72 (t, J = 234.6 Hz), 60.41, 39.44. HRMS (ESI): calculated for C13H13F2N3O2 [M+Na]+: 304.08680, found: 304.08713.

Scheme 1.
Synthesis of key intermediates of pyrazole carboxamide.
2.1.3. Synthesis of Intermediate 4
The intermediate (3, 1 mmol) was dissolved in DMF (5 mL), followed by the addition of triethylamine (1 mmol) and thionyl chloride (3 mmol) at room temperature and stirring for 30 min. The reaction solution was then poured into a saturated sodium bicarbonate solution (100 mL) and extracted thrice using ethyl acetate. The extract was dried over sodium sulfate, filtered, concentrated under reduced pressure, and then subjected to column chromatography (PE/EA = 3/2) to obtain a white product N-(2-(chloromethyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (4, Scheme 1). Intermediate 4, white needle, yield 75%. m.p. 119.9–121.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H, NH), 8.49 (s, 1H, pyrazol-H5), 7.52 (dd, J = 7.6, 1.5 Hz, 1H, Ar-H5), 7.47–7.36 (m, 2H, Ar-H2, H3), 7.33–7.24 (m, 2H, CF2H, Ar-H4), 4.82 (s, 2H, CH2), 3.98 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.25, 145.09 (t, J = 23.4 Hz), 135.77, 133.16, 132.60, 130.38, 129.03, 127.05, 126.29, 115.84, 115.82 (t, J = 3.7 Hz), 109.71 (t, J = 234.7 Hz), 43.27, 39.45. HRMS (ESI): calculated for C13H12ClF2N3O [M+Na]+: 322.05292, found: 322.05263.
2.1.4. General Procedure for the Preparation of Quinazolin-4-Ones [26] (5a1-a32)
Anthranilic acid or its substituted derivatives (10 mmol) were mixed with formamidine acetate (20 mmol) in ethylene glycol monomethyl ether and the reaction mixture was subsequently stirred at 95–130 °C. The mixture was then poured into cold water, thereby precipitating a large amount of solid. The crude product was subsequently obtained by filtration and was dissolved in hot 10% NaOH solution, heated for 5–6 min with charcoal and filtered, and the clear solution was subsequently neutralized (pH = 7) using 1N HCl. The precipitated crystals were filtered out, washed with cooled water, and dried to obtain the quinazolin-4-ones (Scheme 2, Supplementary Materials).

Scheme 2.
Synthesis of key intermediate quinazolinone.
2.1.5. General Procedure for the Preparation of the Target Compounds 6a1-a32
Substituted quinazolinone (1 mmol, 5a1-a32) was added to 10 mL DMF and stirred to dissolve. KOH (59 mg, 1.05) was added and left to react for 40 min, after which 4 (299 mg, 1 mmol) was added. The reaction was carried out at room temperature for 1–3 h. The reaction was detected by TLC, followed by the addition and stirring of 20 mL of saturated ammonium chloride solution for 5 min. It was then extracted with dichloromethane (20 mL × 3), dried over anhydrous sodium sulfate, filtered, concentrated, and purified (Scheme 3).

Scheme 3.
Synthesis of the target compounds 6a1-a32.
3-(difluoromethyl)-1-methyl-N-(2-((4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a1), pale white powder, yield 83%, m.p. 172.8–174.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.21 (s, 1H, NH), 8.54 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.17 (dd, J = 8.0, 1.5 Hz, 1H, H5), 7.84 (ddd, J = 8.4, 7.1, 1.5 Hz, 1H, H7), 7.70 (dd, J = 8.1, 1.1 Hz, 1H, H8), 7.55 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, H6), 7.49 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.35 (td, J = 7.6, 1.8 Hz, 1H, Ar-H3), 7.31 (t, J = 54.3 Hz, 1H, CF2H), 7.28 (dd, J = 7.8, 1.8 Hz, 1H, Ar-H5), 7.23 (dd, J = 7.6, 1.4 Hz, 1H, Ar-H4), 5.23 (s, 2H,CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.63, 160.22, 147.93, 147.90, 145.18 (t, J = 23.1 Hz), 135.36, 134.59, 132.90, 131.20, 128.48, 128.22, 127.28, 127.25, 126.40, 126.20, 126.17, 121.40, 115.91 (t, J = 3.7 Hz), 109.64 (t, J = 234.6 Hz), 59.77, 45.87. HRMS (ESI): calculated for C21H17F2N5O2 [M+Na]+: 432.12425, found: 432.12577.
3-(difluoromethyl)-1-methyl-N-(2-((5-methyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a2), white powder, yield 71%, m.p. 223.1–224.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 1H,NH), 8.55 (s, 1H, pyrazol-H5), 8.44 (s, 1H, H2), 7.65 (t, J = 7.7 Hz, 1H, H7), 7.53–7.47 (m, 2H, H8, Ar-H2), 7.35 (td, J = 7.6, 1.7 Hz, 1H, Ar-H3), 7.32–7.26 (m, 3H, CF2H, H6, Ar-H5), 7.22 (td, J = 7.5, 1.3 Hz, 1H, Ar-H4), 5.17 (s, 2H, CH2), 3.99 (s, 3H, pyrazol-CH3), 2.73 (s, 3H, 5-CH3). 13C NMR (100 MHz, DMSO-d6) δ 161.20, 160.14, 149.54, 147.58, 145.15 (t, J = 23.1 Hz), 140.22, 135.44, 133.67, 132.93, 131.23, 129.60, 128.62, 128.20, 126.15, 126.07, 125.49, 119.85, 115.94 (t, J = 3.7 Hz), 109.61 (t, J =234.7 Hz), 45.60, 39.48, 22.73. HRMS (ESI): calculated for C22H19F2N5O2 [M+Na]+: 446.13990, found: 446.13964.
3-(difluoromethyl)-1-methyl-N-(2-((6-methyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a3), white powder, yield 72%, m.p. 258.8–260.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.21 (s, 1H, NH), 8.54 (s, 1H, pyrazol-H5), 8.43 (s, 1H, H2), 7.99–7.90 (m, 1H, H5), 7.66 (dd, J = 8.4, 2.1 Hz, 1H, H7), 7.60 (d, J = 8.3 Hz, 1H, H8), 7.49 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H2), 7.38–7.31 (m, 1H, Ar-H3), 7.31 (t, J = 54.3 Hz, 1H, CF2H), 7.25 (dd, J = 7.8, 2.0 Hz, 1H, Ar-H5), 7.23–7.18 (m, 1H, Ar-H4), 5.22 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 2.44 (s, 3H, 6-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.54, 160.16, 147.06, 145.96, 145.15 (t, J = 23.1 Hz), 137.11, 135.88, 135.31, 132.87, 131.20, 128.37, 128.17, 127.12, 126.36, 126.14, 125.48, 121.12, 115.89 (t, J = 3.7 Hz), 109.62 (t, J = 234.7 Hz), 45.73, 39.52, 20.82. HRMS (ESI): calculated for C22H19F2N5O2 [M+Na]+: 446.13975, found: 446.13990.
3-(difluoromethyl)-1-methyl-N-(2-((7-methyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a4), white powder, yield 61%, m.p. 246.5–246.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.24 (s, 1H, NH), 8.55 (s, 1H, pyrazol-H5), 8.48 (s, 1H, H2), 8.06 (d, J = 8.1 Hz, 1H, H5), 7.54–7.47 (m, 2H, H6,Ar-H2), 7.38 (dd, J = 8.4, 1.8 Hz, 1H, H8), 7.36–7.32 (m, 1H, Ar-H3), 7.31 (t, J = 54.3 Hz, 1H, CF2H), 7.27 (dd, J = 7.8, 1.7 Hz, 1H, Ar-H5), 7.21 (td, J = 7.5, 1.3 Hz, 1H, Ar-H4), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 2.46 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.54, 160.16, 148.08, 147.94, 145.25, 145.16 (t, J = 23.3 Hz), 135.36, 132.87, 131.10, 128.70, 128.50, 128.21, 126.85, 126.27, 126.09, 126.06, 118.97, 115.91 (t, J = 3.7 Hz), 109.63 (t, J = 234.5 Hz), 45.71, 39.52, 21.31. HRMS (ESI): calculated for C22H19F2N5O2 [M+Na]+: 446.13990, found: 446.13975.
3-(difluoromethyl)-1-methyl-N-(2-((8-methyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a5), white powder, yield 76%, m.p. 215.8–216.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 1H, NH), 8.54 (s, 1H, pyrazol-H5), 8.53 (s, 1H, H2), 8.01 (ddd, J = 8.0, 1.6, 0.7 Hz, 1H, H5), 7.70 (ddd, J = 7.3, 1.6, 0.9 Hz, 1H, H7), 7.49 (dd, J = 8.0, 1.3 Hz, 1H, H6), 7.47–7.42 (m, 1H, Ar-H2), 7.38–7.32 (m, 1H, Ar-H3), 7.31 (t, J = 54.16 Hz, 1H, CF2H), 7.26 (dd, J = 7.8, 1.9 Hz, 1H, Ar-H5), 7.24–7.18 (m, 1H, Ar-H4), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 2.54 (s, 3H, 8-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.85, 160.19, 146.95, 146.41, 145.15 (t, J = 23.2 Hz), 135.52, 135.31, 134.94, 132.89, 131.16, 128.39, 128.19, 126.82, 126.32, 126.15, 123.87, 121.33, 115.89 (t, J = 3.4 Hz), 109.63 (t, J = 234.4 Hz), 45.82, 39.31, 17.03. HRMS (ESI): calculated for C22H19F2N5O2 [M+Na]+: 446.13990, found: 446.13975.
3-(difluoromethyl)-N-(2-((7,8-dimethyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a6), white powder, yield 61%, m.p. 269.0–270.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H, NH), 8.54 (s, 1H, pyrazol-H5), 8.51 (s, 1H, H2), 7.93 (d, J = 8.1 Hz, 1H, H5), 7.50 (dd, J = 8.0, 1.3 Hz, 1H, H6), 7.37 (d, J = 8.2 Hz, 1H, Ar-H2), 7.33 (dd, J = 7.9, 1.8 Hz, 1H, Ar-H3), 7.30 (t, J = 54.1 Hz, 1H, CF2H), 7.26 (dd, J = 7.8, 1.7 Hz, 1H, Ar-H5), 7.24–7.18 (m, 1H, Ar-H4), 5.20 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 2.48 (s, 3H, 8-CH3), 2.40 (s, 3H, 7-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.92, 160.15, 146.71, 146.12, 145.15 (t, J = 23.5 Hz), 143.28, 135.33, 133.50, 132.87, 131.06, 128.99, 128.44, 128.19, 126.20, 126.08, 123.05, 119.24, 115.92 (t, J = 3.6 Hz), 109.63 (t, J = 234.7 Hz), 45.62, 39.52, 20.46, 12.77. HRMS (ESI): calculated for C23H21F2N5O2 [M+Na]+: 460.15555, found: 460.15515.
3-(difluoromethyl)-N-(2-((5-fluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a7), white powder, yield 66%, m.p. 243.1–244.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.11 (s, 1H, NH), 8.52 (s, 1H, pyrazol-H5), 8.45 (s, 1H, H2), 7.81 (td, J = 8.2, 5.5 Hz, 1H, H7), 7.50 (dd, J = 8.3, 1.0 Hz, 1H, H8), 7.44 (dd, J = 8.0, 1.3 Hz, 1H, H6), 7.38–7.29 (m, 2H, Ar-H2, Ar-H3), 7.28 (t, J = 54.1 Hz, 1H, CF2H), 7.27–7.20 (m, 2H, Ar-H4, Ar-H5), 5.17 (s, 2H, CH2), 3.99 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) 160.34 (d, J = 263.6 Hz, 1H), 160.26, 159.03, 157.54 (d, J = 3.8 Hz, 1H), 157.52, 150.09, 148.88, 145.13 (t, J = 23.0 Hz), 135.33, 135.24 (d, J = 10.6 Hz, 1H), 132.22 (d, J = 153.8 Hz, 1H), 128.40, 128.17, 126.45, 123.36 (d, J = 33.7 Hz, 1H), 115.81 (t, J = 3.6 Hz), 113.60 (d, J = 20.6 Hz, 1H), 111.11 (d, J = 5.8 Hz, 1H), 109.60 (t, J = 235.3 Hz), 45.73, 39.49. HRMS (ESI): calculated for C21H16F3N5O2 [M+Na]+: 450.11483, found: 450.11509.
3-(difluoromethyl)-N-(2-((6-fluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a8), white powder, yield 59%, m.p. 235.4–237.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.19 (s, 1H, NH), 8.53 (s, 1H, pyrazol-H5), 8.48 (s, 1H, H2), 7.84 (dd, J = 8.7, 2.8 Hz, 1H, H5), 7.81–7.70 (m, 2H, H7), 7.46 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.39–7.31 (m, 2H, H8, Ar-H3), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.26–7.18 (m, 2H, Ar-H4, Ar-H5), 5.22 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.35 (d, J = 245.6 Hz), 160.22, 159.97 (d, J = 3.2 Hz), 159.13, 154.86, 147.38, 145.15 (t, J = 23.0 Hz), 144.83, 135.36, 132.91, 131.14, 130.8 (d, J = 8.4 Hz), 128.41 (d, J = 28.2 Hz), 126.39 (d, J = 32.0 Hz), 123.10 (d, J = 24.1 Hz), 122.71 (d, J = 8.7 Hz), 115.83 (t, J = 3.7 Hz), 110.86 (d, J = 23.5 Hz), 109.60 (t, J = 235.3 Hz), 45.98, 39.49. HRMS (ESI): calculated for C21H16F3N5O2 [M+Na]+: 450.11483, found: 450.11509.
3-(difluoromethyl)-N-(2-((7-fluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a9), white powder, yield 62%, m.p. 229.8–230.4 °C. 1H NMR (400 MHz, DMSO) δ 10.18 (s, 1H, NH), 8.53 (s, 1H, pyrazol-H5), 8.52 (s, 1H, H2), 8.24–8.11 (m, 2H, H5,H6), 7.51–7.45 (m, 1H, H8), 7.42 (d, J = 2.6 Hz, 1H, Ar-H2), 7.36–7.32 (m, 1H, Ar-H3), 7.29 (t, J = 54.1 Hz, 1H, CF2H), 7.27 (dd, J = 7.7, 1.8 Hz, 1H, Ar-H5), 7.22 (td, J = 7.4, 1.3 Hz, 1H, Ar-H4), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 165.71 (d, J = 251.6 Hz), 160.22, 159.92, 150.11 (d, J = 13.2 Hz), 149.32, 146.94, 145.16 (t, J = 23.2 Hz), 135.34, 132.91, 131.22, 129.40 (d, J = 10.9 Hz), 128.36(d, J = 24.3 Hz), 126.54, 126.23, 118.49, 115.88 (d, J = 23.6 Hz), 115.85 (t, J = 3.6 Hz), 112.30 (d, J = 21.5 Hz),109.61 (t, J = 234.7 Hz), 45.93, 39.48. HRMS (ESI): calculated for C21H16F3N5O2 [M+Na]+: 450.11483, found: 450.11509.
3-(difluoromethyl)-N-(2-((8-fluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a10), white powder, yield 69%, m.p. 274.8–276.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, NH), 8.51 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 7.95 (dd, J = 8.5, 1.3 Hz, 1H, H5), 7.76–7.69 (m, 1H, H7), 7.57–7.52 (m, 1H, Ar-H6), 7.44 (dd, J = 7.8, 1.2 Hz, 1H, Ar-H2), 7.38–7.31 (m, 1H, Ar-H3), 7.27(t, J = 54.1 Hz, 1H, CF2H), 7.27–7.20 (m, 2H, Ar-H4, Ar-H5), 5.22 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.25, 159.69 (d, J = 3.3 Hz), 156.41 (d, J = 254.3 Hz), 148.56, 145.15 (t, J = 23.2 Hz), 137.16 (d, J = 12.3 Hz), 135.29, 132.92, 131.32, 128.34, 128.24, 127.65 (d, J = 7.9 Hz), 126.63, 126.32, 123.49, 121.95 (d, J = 4.2 Hz), 119.97 (d, J = 16.0 Hz), 115.82 (t, J = 3.6 Hz), 109.61 (t, J = 234.7 Hz), 46.12, 39.72. HRMS (ESI): calculated for C21H16F3N5O2 [M+Na]+: 450.11483, found: 450.11509.
3-(difluoromethyl)-N-(2-((6,7-difluoro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a11), white powder, yield 69%, m.p. 243.1–244.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.15 (s, 1H, NH), 8.52 (s, 1H, pyrazol-H5), 8.52 (s, 1H, H2), 8.06 (dd, J = 10.4, 8.6 Hz, 1H, H5), 7.78 (dd, J = 11.3, 7.3 Hz, 1H, H8), 7.46 (dd, J = 8.1, 1.3 Hz, 1H, Ar-H2), 7.35 (td, J = 7.5, 1.7 Hz, 1H, Ar-H3), 7.32–7.28 (m, 1H, Ar-H5), 7.28(t, J = 54.1 Hz, 1H, CF2H), 7.23 (td, J = 7.5, 1.3 Hz, 1H, Ar-H4), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.22, 159.37 (d, J = 2.9 Hz), 153.89 (dd, J = 54.6, 14.6 Hz), 148.89 (dd, J = 249.1, 14.2 Hz), 148.80 (d, J = 2.0 Hz), 145.16 (t, J = 23.1 Hz), 135.37, 132.88, 131.05, 128.61, 128.33, 126.59, 126.24, 118.81 (dd, J = 6.7, 2.0 Hz), 115.80 (t, J = 3.6 Hz), 115.31 (d, J = 17.7 Hz), 113.78 (d, J = 19.4 Hz), 109.59 (t, J = 234.7 Hz), 46.09, 39.72. HRMS (ESI): calculated for C21H16F4N5O2 [M+Na]+: 468.10541, found: 468.10521.
N-(2-((5-chloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a12), white powder, yield 58%, m.p. 172.8–174.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.14 (s, 1H, NH), 8.53 (s, 1H, pyrazol-H5), 8.49 (s, 1H, H2), 7.78–7.70 (m, 1H, H7), 7.62 (dt, J = 8.2, 1.4 Hz, 1H, H6), 7.56 (dq, J = 7.8, 1.2 Hz, 1H, H8), 7.46 (dt, J = 8.0, 1.4 Hz, 1H, Ar-H2), 7.42–7.29 (m, 2H, CF2H, Ar-H3), 7.28–7.11 (m, 2H, Ar-H4, Ar-H5), 5.16 (s, 2H, CH2), 3.99 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.22, 158.57, 150.42, 148.65, 145.14 (t, J = 23.0 Hz), 135.44, 134.37, 132.98, 132.50, 131.25, 129.71, 128.69, 128.27, 126.92, 126.44, 126.21, 118.50, 115.83 (t, J = 3.7 Hz), 109.59 (t, J = 234.7 Hz), 46.08, 39.70. HRMS (ESI): calculated for C21H16ClF2N5O2 [M+Na]+: 446.08528, found: 466.08523.
N-(2-((6-chloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a13), white powder, yield 66%, m.p. 259.1–260.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H, NH), 8.69 (s, 1H, pyrazol-H5), 8.52 (s, 1H, H2), 8.07 (d, J = 2.5 Hz, 1H, H5), 7.85 (dd, J = 8.8, 2.5 Hz, 1H, H7), 7.71 (d, J = 8.7 Hz, 1H, H8), 7.43 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.36–7.31 (m, 1H, Ar-H3), 7.26(t, J = 54.1 Hz, 1H, CF2H), 7.25–7.18 (m, 2H, Ar-H4, Ar-H5), 5.26 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.29, 159.56, 148.40, 146.63, 145.15 (t, J = 23.0 Hz), 135.39, 134.63, 133.15, 131.52, 131.31, 130.98, 128.51, 128.16, 126.86, 126.20, 125.13, 122.72, 115.81 (t, J = 3.7 Hz), 109.59 (t, J = 234.7 Hz), 46.14, 39.52. HRMS (ESI): calculated for C21H16ClF2N5O2 [M+Na]+: 446.08528, found: 466.08523.
N-(2-((7-chloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a14), white powder, yield 63%, m.p. 250.3–252.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.14 (s, 1H, NH), 8.51 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.14 (d, J = 8.6 Hz, 1H, H5), 7.76 (d, J = 2.1 Hz, 1H, H8), 7.59 (dd, J = 8.5, 2.1 Hz, 1H, H6), 7.45 (dd, J = 7.9, 1.3 Hz, 1H, Ar-H2), 7.35 (td, J = 7.5, 1.9 Hz, 1H, Ar-H3),7.28 (t, J = 54.1 Hz, 1H, CF2H), 7.27–7.19 (m, 2H, Ar-H4, Ar-H5), 5.20 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.22, 160.00, 149.38, 149.01, 145.13 (t, J = 23.2 Hz), 139.21, 135.31, 132.88, 131.24, 128.45, 128.29, 128.26, 127.55, 126.59, 126.42, 126.27, 120.29, 115.82(t, J = 3.6 Hz), 109.60 (t, J = 234.6 Hz), 46.02, 39.75. HRMS (ESI): calculated for C21H16ClF2N5O2 [M+Na]+: 446.08528, found: 466.08523.
N-(2-((8-chloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a15), white powder, yield 68%, m.p. 231.8–233.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, NH), 8.58 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.10 (dd, J = 7.7, 1.2 Hz, 1H, H5), 7.99 (dd, J = 7.8, 1.4 Hz, 1H, H7), 7.56–7.50 (m, 1H, H6), 7.44 (dd, J = 7.9, 1.3 Hz, 1H, Ar-H2), 7.35 (ddd, J = 8.1, 6.7, 2.2 Hz, 1H, Ar-H3), 7.29(t, J = 54.1 Hz, 1H, CF2H), 7.28–7.20 (m, 2H, Ar-H4, Ar-H5), 5.22 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.25, 160.01, 148.86, 145.24, 145.15 (t, J = 23.2 Hz), 144.40, 135.27, 134.62, 132.93, 131.28, 128.31, 128.23, 127.65, 126.62, 126.33, 125.37, 123.20, 115.80 (t, J = 3.7 Hz), 109.61 (t, J = 235.4 Hz), 46.17, 39.72. HRMS (ESI): calculated for C21H16ClF2N5O2 [M+Na]+: 446.08528, found: 466.08523.
N-(2-((6,8-dichloro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a16), white powder, yield 77%, m.p. 294.1–294.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 1H, NH), 8.60 (s, 1H, pyrazol-H5), 8.59 (s, 1H, H2), 8.12 (d, J = 2.4 Hz, 1H, H5), 7.41 (dd, J = 8.1, 1.5 Hz, 1H, H7), 7.34 (td, J = 7.4, 2.0 Hz, 1H, Ar-H2), 7.27–7.20 (m, 3H, CF2H, Ar-H4, Ar-H5) 5.23 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.29, 159.61, 150.97, 149.19, 147.16, 146.02 (t, J = 23.2 Hz), 144.33, 133.86, 132.30, 131.21, 130.53, 128.41, 128.23, 127.44, 125.02, 124.43, 124.15, 116.16 (t, J = 3.7 Hz), 108.33 (t, J = 235.7 Hz), 55.69, 39.52. HRMS (ESI): calculated for C21H15Cl2F2N5O2 [M+Na]+: 500.04631, found: 500.04620.
N-(2-((6-chloro-8-methyl-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a17), white powder, yield 69%, m.p. >310 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.29 (s, 1H, NH), 8.62 (s, 1H, pyrazol-H5), 8.55 (s, 1H, H2), 7.92 (dd, J = 2.6, 0.7 Hz, 1H, H5), 7.76 (dd, J = 2.5, 1.0 Hz, 1H, H7), 7.44 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H2), 7.36–7.31 (m, 1H, Ar-H3),7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.26–7.18 (m, 2H, Ar-H4, Ar-H5), 5.23 (s, 2H, CH2), 3.99 (s, 3H, pyrazol-CH3), 2.51 (s, 3H, 8-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.25, 159.82, 147.45, 145.27, 145.14 (t, J = 23.2 Hz), 138.57, 135.33, 134.54, 133.07, 131.24, 130.98, 128.43, 128.17, 126.73, 126.22, 122.65, 122.57, 115.81 (t, J = 3.4 Hz), 109.60 (t, J = 235.3 Hz), 46.10, 39.73. 16.77. HRMS (ESI): calculated for C22H18ClF2N5O2 [M+Na]+: 480.10093, found:480.10045.
N-(2-((5-bromo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a18), gray powder, yield 69%, m.p. 274.8–276.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.17 (s, 1H, NH), 8.55 (s, 1H, pyrazol-H5), 8.52 (s, 1H, H2), 7.78 (dd, J = 6.6, 2.3 Hz, 1H, H6), 7.70–7.62 (m, 2H, H7, H8), 7.47 (dd, J = 7.9, 1.4 Hz, 1H, Ar-H2), 7.38–7.31 (m, 2H, Ar-H3, Ar-H5),7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.24 (td, J = 7.5, 1.4 Hz, 1H, Ar-H4), 5.16 (s, 2H, CH2), 3.99 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.18, 158.72, 150.26, 148.43, 145.13 (t, J = 23.2 Hz), 135.46, 134.68, 133.52, 132.94, 131.07, 128.78, 128.31, 127.61, 126.33, 126.16, 120.15, 119.37, 115.84 (t, J = 3.6 Hz), 109.59 (t, J = 234.7 Hz), 46.21, 39.54. HRMS (ESI): calculated for C21H16BrF2N5O2 [M+Na]+: 510.03476, found: 510.03443.
N-(2-((6-bromo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a19), white powder, yield 41%, m.p. 281.6–282.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, NH), 8.51 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.23 (d, J = 2.4 Hz, 1H, H5), 7.99 (dd, J = 8.7, 2.4 Hz, 1H, H7), 7.65 (d, J = 8.7 Hz, 1H, H8), 7.44 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.35 (td, J = 7.5, 2.0 Hz, 1H, Ar-H3), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.26–7.20 (m, 2H, Ar-H4, Ar-H5), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.22, 159.46, 148.50, 146.92, 145.13 (t, J = 23.0 Hz), 144.90, 137.42, 135.30, 132.88, 131.23, 129.67, 128.45, 128.26, 126.65, 126.29, 123.05, 119.77, 115.79(t, J = 3.4 Hz), 109.59(t, J = 235.3 Hz), 46.08, 39.73. HRMS (ESI): calculated for C21H16BrF2N5O2 [M+Na]+: 510.03476, found: 510.03443.
N-(2-((7-bromo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a20), grey powder, yield 67%, m.p. 262.8–263.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.14 (s, 1H, NH), 8.50 (s, 1H, pyrazol-H5), 8.49 (s, 1H, H2), 8.05 (dd, J = 8.5, 1.1 Hz, 1H, H5), 7.95–7.87 (m, 1H, H6), 7.75–7.67 (m, 1H, H8), 7.45 (dd, J = 8.0, 1.9 Hz, 1H, Ar-H2), 7.38–7.31 (m, 1H Ar-H3), 7.30–7.12 (m, 3H, CF2H, Ar-H4, Ar-H5), 5.20 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.22, 160.14, 149.32, 149.05, 145.14 (t, J = 23.2 Hz), 135.31, 132.89, 131.22, 130.30, 129.51, 128.46, 128.26, 128.26, 128.18, 126.59, 126.28, 120.58, 115.85 (d, J = 3.7 Hz), 109.60 (t, J = 234.6 Hz), 46.04, 39.73. HRMS (ESI): calculated for C21H16BrF2N5O2 [M+Na]+: 510.03476, found: 510.03443.
N-(2-((8-bromo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a21), white powder, yield 59%, m.p. 263.7–266.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, NH), 8.58 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.18–8.13 (m, 2H, H5, H7), 7.49–7.43 (m, 2H, H6, Ar-H2), 7.39–7.31 (m, 1H, Ar-H3), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.28–7.20 (m, 2H, Ar-H4, Ar-H5), 5.21 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.26, 160.00, 148.93, 145.42 (t, J = 23.2 Hz), 145.14, 137.95, 135.27, 132.93, 131.28, 128.30, 128.23, 128.12, 126.61, 126.33, 126.07, 123.10, 121.81, 115.80(t, J = 3.6 Hz), 109.61 (t, J = 234.7 Hz), 46.18, 39.73. HRMS (ESI): calculated for C21H16BrF2N5O2 [M+Na]+: 510.03476, found: 510.03443.
N-(2-((6,8-dibromo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide (6a22), white powder, yield 57%, m.p. >310 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H, NH), 8.60 (s, 1H, pyrazol-H5), 8.48 (s, 1H, H2), 8.38 (d, J = 2.2 Hz, 1H, H5), 8.20 (d, J = 2.2 Hz, 1H, H7), 7.41 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.35 (ddd, J = 8.0, 6.7, 2.0 Hz, 1H, Ar-H3), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.26–7.20 (m, 2H, Ar-H4, Ar-H5), 5.20 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.24, 158.95, 149.35, 145.12 (t, J = 23.2 Hz), 144.70, 139.68, 135.24, 132.91, 131.22, 128.36, 128.28, 128.15, 126.80, 126.39, 124.05, 123.33, 119.55, 115.72 (t, J = 3.7 Hz), 109.57 (t, J = 235.7 Hz), 46.39, 39.72. HRMS (ESI): calculated for C21H16Br2F2N5O2 [M+Na]+: 587.94528, found: 587.94462.
3-(difluoromethyl)-N-(2-((5-methoxy-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a23), white powder, yield 68%, m.p. 212.9–214.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.24 (s, 1H, NH), 8.57 (s, 1H, pyrazol-H5), 8.42 (s, 1H, H2), 7.72 (t, J = 8.2 Hz, 1H, H7), 7.50 (dd, J = 8.0, 1.4 Hz, 1H, Ar-H2), 7.34 (td, J = 7.6, 1.7 Hz, 1H, Ar-H3), 7.30 (t, J = 54.1 Hz, 1H, CF2H), 7.29–7.26 (m, 1H, Ar-H5), 7.24–7.17 (m, 2H, H8, Ar-H4), 7.05 (dd, J = 8.4, 1.0 Hz, 1H, H6), 5.12 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 3.85 (s, 3H, 5-CH3O). 13C NMR (100 MHz, DMSO-d6) δ 160.16, 159.71, 158.51, 150.49, 148.22, 145.37 (t, J = 23.2 Hz), 135.44, 135.01, 132.96, 131.14, 128.66, 128.15, 125.99, 124.39, 119.00, 115.92 (t, J = 3.7 Hz), 109.78 (t, J = 235.4 Hz), 56.13, 45.57, 39.72. HRMS (ESI): calculated for C22H19F2N5O3 [M+Na]+: 439.14560, found: 462.13426.
3-(difluoromethyl)-N-(2-((6-methoxy-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a24), white powder, yield 70%, m.p. 259.8–260.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.30 (s, 1H, NH), 8.64 (s, 1H, pyrazol-H5), 8.38 (s, 1H, H2), 7.64 (d, J = 8.9 Hz, 1H, H5), 7.52 (d, J = 3.0 Hz, 1H, H8), 7.50–7.43 (m, 2H, H6 Ar-H2), 7.38–7.31 (m, 1H, Ar-H3), 7.30 (t, J = 54.1 Hz, 1H, CF2H), 7.27–7.17 (m, 2H, Ar-H4, Ar-H5), 5.25 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3), 3.86 (s, 3H, 6-CH3O). 13C NMR (100 MHz, DMSO-d6) δ 160.32, 160.22, 158.12, 145.67, 145.15 (t, J = 23.2 Hz), 135.35, 133.07, 131.34, 128.96, 128.40, 128.11, 126.56, 126.13, 124.04, 122.28, 115.89 (t, J = 3.7 Hz), 109.63 (t, J = 235.4 Hz), 106.10, 55.66, 45.84, 39.52. HRMS (ESI): calculated for C22H19F2N5O3 [M+Na]+: 439.14560, found: 462.13426.
3-(difluoromethyl)-N-(2-((7-methoxy-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a25), white powder, yield 69%, m.p. 254.1–276.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.43 (s, 1H, NH), 8.70 (s, 1H, pyrazol-H5), 8.50 (s, 1H, H2), 8.07 (d, J = 9.6 Hz, 1H, H5), 7.49 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.33 (td, J = 7.5, 1.8 Hz, 1H, Ar-H3), 7.30 (t, J = 54.1 Hz, 1H, CF2H), 7.26 (dd, J = 7.8, 1.7 Hz, 1H, Ar-H5), 7.20 (td, J = 7.5, 1.3 Hz, 1H, Ar-H4), 7.15–7.11 (m, 2H, H6, H8), 5.23 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3), 3.89 (s, 3H, 7-CH3O). 13C NMR (100 MHz, DMSO-d6) δ 164.15, 160.20, 160.18, 150.26, 148.52, 145.16 (t, J = 23.2 Hz), 135.41, 133.09, 131.18, 128.55, 128.11, 127.85, 126.42, 126.01, 116.86, 115.93 (t, J = 3.7 Hz), 114.80, 109.64 (t, J = 235.4 Hz), 108.29, 55.82, 45.66, 39.52. HRMS (ESI): calculated for C22H19F2N5O3 [M+Na]+: 439.14560, found: 462.13426.
3-(difluoromethyl)-N-(2-((8-methoxy-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a26), white powder, yield 70%, m.p. 268.8–270.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 1H, NH), 8.54 (s, 1H, pyrazol-H5), 8.44 (s, 1H, H2), 7.71 (dd, J = 8.0, 1.3 Hz, 1H, H5), 7.52–7.43 (m, 2H, H6, Ar-H2), 7.41–7.32 (m, 2H, H7, Ar-H3), 7.31 (t, J = 54.1 Hz, 1H, CF2H), 7.29–7.17 (m, 2H, Ar-H4, Ar-H5), 5.22 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 3.90 (s, 3H, 8-CH3O). 13C NMR (100 MHz, DMSO-d6) δ 160.51, 160.17, 154.53, 146.49, 145.16 (t, J = 23.2 Hz), 138.45, 135.33, 132.90, 131.14, 128.35, 128.18, 127.68, 126.34, 126.14, 122.46, 117.07, 115.89 (t, J = 3.7 Hz), 115.08, 109.65 (t, J = 235.4 Hz), 56.01, 45.82, 39.52. HRMS (ESI): calculated for C22H19F2N5O3 [M+Na]+: 439.14560, found: 462.13426.HRMS (ESI): calculated for C22H19F2N5O3 [M+Na]+: 439.14560, found: 462.13426.
3-(difluoromethyl)-N-(2-((6,7-dimethoxy-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a27), white powder, yield 77%, m.p. 290.3–292.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H, NH), 8.55 (s, 1H, pyrazol-H5), 8.41 (s, 1H, H2), 7.51 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H2), 7.46 (s, 1H, H5), 7.34 (td, J = 7.6, 1.8 Hz, 1H, Ar-H3), 7.31 (t, J = 54.1 Hz, 1H, CF2H), 7.26 (dd, J = 7.8, 1.8 Hz, 1H, Ar-H5), 7.21 (ddd, J = 7.8, 7.1, 1.3 Hz, 1H, Ar-H4), 7.16 (s, 1H, H8), 5.21 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3), 3.91 (s, 3H, 7-CH3O), 3.87 (s, 3H, 6-CH3O). 13C NMR (100 MHz, DMSO-d6) δ 160.13, 159.94, 154.80, 149.00, 146.37, 145.15 (t, J = 23.2 Hz), 144.22, 135.36, 132.90, 131.05, 128.54, 128.20, 126.17, 126.03, 115.95 (t, J = 3.7 Hz), 114.40, 109.64 (t, J = 235.7 Hz), 107.92, 105.12, 56.05, 55.76, 45.68, 39.72. HRMS (ESI): calculated for C23H21F2N5O4 [M+Na]+: 492.14538, found: 492.14539.
3-(difluoromethyl)-1-methyl-N-(2-((5-nitro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a28), white powder, yield 75%, m.p. 278.1–279.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, NH), 8.61 (s, 1H, H2), 8.43 (s, 1H, pyrazol-H5), 8.02–7.98 (m, 1H, H6), 7.94–7.91 (m, 1H, H8), 7.83–7.79 (m, 1H, H7), 7.46 (dd, J = 7.9, 1.3 Hz, 1H, Ar-H2), 7.39–7.32 (m, 2H, Ar-H3, Ar-H5), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.24 (td, J = 7.5, 1.4 Hz, 1H, Ar-H4), 5.18 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.24, 157.45, 157.18, 149.69, 148.15, 145.15(t, J = 23.2 Hz), 135.50, 134.99, 132.91, 130.75, 130.15, 130.04, 128.92, 128.48, 126.42, 126.24, 120.99, 115.76(t, J = 3.6 Hz), 109.58 (t, J = 234.7 Hz), 46.17, 39.47. HRMS (ESI): calculated for C21H16F2N6O4 [M+Na]+: 477.10933, found: 477.10936.
3-(difluoromethyl)-1-methyl-N-(2-((6-nitro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a29), light yellow powder, yield 64%, m.p. 233.9–234.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.07 (s, 1H, NH), 8.77 (d, J = 2.7 Hz, 1H, H5), 8.63 (s, 1H, pyrazol-H5), 8.55 (dd, J = 9.0, 2.7 Hz, 1H, H7), 8.49 (s, 1H, H2), 7.88 (d, J = 9.0 Hz, 1H, H8), 7.40 (dd, J = 7.9, 1.6 Hz, 1H, Ar-H2), 7.36 (td, J = 7.2, 1.8 Hz, 1H, Ar-H3), 7.31–7.27 (m, 1H, Ar-H5), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.27–7.22 (m, 1H, Ar-H4), 5.25 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.29, 159.73, 151.97, 151.22, 145.31, 145.11 (t, J = 23.2 Hz), 135.28, 132.92, 131.45, 129.15, 128.45, 128.39, 128.33, 127.07, 126.48, 122.22, 121.64, 115.68 (t, J = 3.7 Hz), 109.53 (t, J = 235.7 Hz), 46.35, 39.52. HRMS (ESI): calculated for C21H16F2N6O4 [M+Na]+: 477.10933, found: 477.10936.
3-(difluoromethyl)-1-methyl-N-(2-((7-nitro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a30), orange powder, yield 61%, m.p. 253.8–256.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, NH), 8.60 (s, 1H, pyrazol-H5), 8.48 (s, 1H, H2), 8.41 (d, J = 2.2 Hz, 1H, H8), 8.34 (dd, J = 8.8, 0.5 Hz, 1H, H5), 8.26 (dd, J = 8.8, 2.3 Hz, 1H, H6), 7.41 (dd, J = 7.9, 1.4 Hz, 1H, Ar-H2), 7.36 (td, J = 7.4, 1.9 Hz, 1H, Ar-H3), 7.28 (dd, J = 7.8, 1.9 Hz, 1H, Ar-H5), 7.26–7.21 (m, 2H, CF2H, Ar-H4), 5.23 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.29, 159.59, 151.14, 150.16, 148.27, 145.12 (t, J = 23.2 Hz), 135.30, 132.91, 131.35, 128.53, 128.43, 128.31, 126.88, 126.42, 125.81, 122.33, 120.83, 115.78 (t, J = 3.7 Hz), 109.57 (t, J = 235.7 Hz), 46.35, 39.72. HRMS (ESI): calculated for C21H16F2N6O4 [M+Na]+: 477.10933, found: 477.10936.
3-(difluoromethyl)-1-methyl-N-(2-((8-nitro-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1H-pyrazole-4-carboxamide (6a31), yellow powder, yield 60%, m.p. 226.8–208.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, NH), 8.59 (s, 1H, pyrazol-H5), 8.48 (s, 1H, H2), 8.36 (d, J = 8.0 Hz, 1H, H5), 8.33 (d, J = 7.9 Hz, 1H, H7), 7.69 (t, J = 7.9 Hz, 1H, H6), 7.46–7.38 (m, 1H, Ar-H2), 7.37–7.32 (m, 1H, Ar-H3), 7.31–7.20 (m, 3H, CF2H, Ar-H4, Ar-H5), 5.22 (s, 2H, CH2), 4.00 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.29, 159.13, 150.56, 146.48, 145.14 (t, J = 23.2 Hz), 139.66, 135.26, 132.95, 131.35, 129.96, 128.39, 128.28, 127.98, 126.94, 126.83, 126.44, 122.96, 115.74 (t, J = 3.7 Hz), 109.59 (t, J = 235.7 Hz), 46.43, 39.52. HRMS (ESI): calculated for C21H16F2N6O4 [M+Na]+: 477.10933, found: 477.10936.
3-(difluoromethyl)-N-(2-((6-iodo-4-oxoquinazolin-3(4H)-yl)methyl)phenyl)-1-methyl-1H-pyrazole-4-carboxamide (6a32), white powder, yield 74%, m.p. 261.8–263.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H, NH), 8.49 (s, 1H, pyrazol-H5), 8.49 (s, 1H, H2), 8.41 (d, J = 2.1 Hz, 1H, H5), 8.14–8.07 (m, 1H, H7), 7.50–7.44 (m, 2H, H8, Ar-H2), 7.39–7.30 (m, 1H, Ar-H3), 7.27 (t, J = 54.1 Hz, 1H, CF2H), 7.26–7.19 (m, 2H, Ar-H5, Ar-H5), 5.20 (s, 2H, CH2), 4.01 (s, 3H, pyrazol-CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.21, 159.26, 148.53, 147.19, 145.13(t, J = 23.2 Hz), 142.92, 135.27, 134.40, 132.88, 131.27, 129.40, 128.39, 128.24, 126.65, 126.29, 123.20, 115.80 (t, J = 3.7 Hz), 109.59 (t, J = 235.7 Hz), 92.48, 46.06, 39.73. HRMS (ESI): calculated for C21H16F2IN5O2 [M+Na]+: 558.02090, found: 558.02095.
2.2. X-ray Diffraction
Compound 6a11 was recrystallized through slow evaporation from a tetrahydrofuran/N-hexane (υ/υ = 2:1) solution to produce a single crystal that was suitable for X-ray crystallography for structure validation.
2.3. In Vitro Antifungal Assay
R. solani was the test strain. It was provided by the Guizhou Institute of Plant Protection. In this study, the in vitro antifungal activity of the target compound 6a1-a32 against R. solani was screened using the mycelial growth rate method [27,28]. The commercial fungicides fluconazole and bixafen were selected as the positive controls. R. solani was inoculated on potato dextrose agar (PDA) plates and grown in biochemical incubators at 28 ± 1 °C for 2 days. The newly grown mycelia were used to determine the antifungal activity. The tested compounds were dissolved in DMSO to prepare 10 mg/mL stock solutions before mixing with PDA. The PDA containing the test compounds at a concentration of 100 mg/L was then poured into sterile Petri dishes for primary screening. A data processing system (DPS, V9.50) was used for statistical analysis of the test data, and the significant differences were determined using Duncan’s new multiple range method. The EC50 values and 95% confidence limits were calculated after testing the inhibition rates, based on Duncan’s new multiple range method. The inhibition rate of the potent compounds was further tested and the corresponding EC50 values were calculated using DPS. The relative inhibitory rates of the potent compounds were then calculated using the following equation:
where C is the colony diameter of the control (mm), T is the colony diameter of the treatment (mm), and 5 is the diameter of the mycelium disks.
Inhibition rate (%) = [(C − T)/(C − 5)] × 100%
2.4. SEM Observations
SEM observations of R. solani hyphae were conducted following the reported methods [29,30]. Mycelium disks with a diameter of 5 mm were taken from the edge of the PDA medium containing 12.29 mg/L of 6a16 and were incubated for 2 days at 28 ± 1 °C. Mycelium disks from PDA with 0.1% DMSO were used as controls. The samples were then fixed at 4 °C using 2.5% glutaraldehyde for 1 day and were subsequently rinsed thrice with 0.1 M phosphate buffer for 15 min. The samples were then fixed with 1% OsO4 solution for 1 h and then dehydrated in 10%, 30%, 50%, 70%, 90%, and 100% ethanol at 10 min intervals. Gold coating of the samples was then carried out after drying at the critical point, followed by SEM observations.
3. Result
3.1. Chemistry
Hybrid molecules are defined as chemical entities with two or more structural domains having different biological functions and dual activity, indicating that a hybrid molecule acts as two distinct pharmacophores. Hybrid antifungal molecules 6a1-a32, based on the conjugation of quinazolinone to pyrazolecarbamide, were designed and synthesized. Scheme 1, Scheme 2 and Scheme 3 show the synthetic route of target compounds 6a1-a32. First, 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid 1 was reacted with oxalyl chloride to obtain pyrazole-4-carbonyl chloride 2, a light yellow, oily liquid [25]. Intermediate 3 (a white product) was obtained by reacting 2 with o-aminobenzyl alcohol in a saturated sodium carbonate solution. Intermediate 3 was subsequently chlorinated by dichlorosulfoxide at room temperature to yield intermediate 4, a white solid (Scheme 1). Optimized preparative procedures for all quinazolin-4-ones 5a1-a32 were conducted following László Örfi [26]. Anthranilic acid or its substituted derivatives were mixed with formamidine acetate in ethylene glycol monomethyl ether at 95–130 °C to obtain quinazolin-4-ones (Scheme 2). The target compounds of quinazolinone-scaffold-containing pyrazole carboxamides were finally obtained by reacting intermediate 4 with different substituted quinazolin-4-ones 5a1-a32 in DMF under alkaline conditions (Scheme 3). The structures of all key intermediates and target compounds were confirmed using 1H and 13C NMR and HRMS, and their spectra data are shown in the Supplementary Materials.
3.2. X-ray Diffraction
To further validate the structure of the target compounds, the structure of 6a11 was further identified by X-ray diffraction studies (Figure 4).

Figure 4.
Crystal structure for 6a11 (CCDC number: 2218016).
3.3. In Vitro Antifungal Assay
Table 1 and Table 2 outline the preliminary in vitro antifungal activities of the 32 target compounds at 100 mg/L. Most of the target compounds exhibited some degree of antifungal activity, with inhibition rates ranging between 13.63% and 100% against R. solani (Table 1). Notably, compounds 6a24 and 6a29 showed good antifungal activity against R. solani, with inhibition rates of 70.26% and 70.53%, respectively. Nonetheless, these rates were lower than those of fluconazole (100%) and bixafen (100%). Hybrid antifungal molecule 6a16 exhibited the best antifungal activity against R. solani, matching that of fluconazole and bixafen.

Table 1.
Inhibition effect in vitro of target compounds 6a1-a32 at 100 mg/L against R. solani.

Table 2.
EC50 values of 6a16 against R. solani.
Figure 5 shows the results of the structure–activity relationship analysis. The trend of the inhibitory activity against R. solani was highest when the quinazolinone of the target compounds was substituted with a bromine atom at substitution site 6. Notably, the same trend of antifungal activity was maintained when F-, Cl-, and CH3O- were substituted at different sites with a bromine atom. The fungal activity inhibition trend was 6-CH3O > 6-Cl > 6-Br- > 6-F > 6-CH3 when the substitution site was at position 6. In addition, the number and position of the substituent atoms affected the antifungal activity. For example, the structural differences of 6a12, 6a13, 6a14, 6a15, and 6a16 were at different positions and quantities of chlorine atoms, and their inhibition rates were 41.43%, 61.93%, 45.90%, 45.43%, and 100%, respectively. The antifungal activity at 6,8-diCl > 6-Cl > 7-Cl≈8-Cl > 5-Cl indicated that the position and number of chlorine atoms directly affected the antifungal activity.
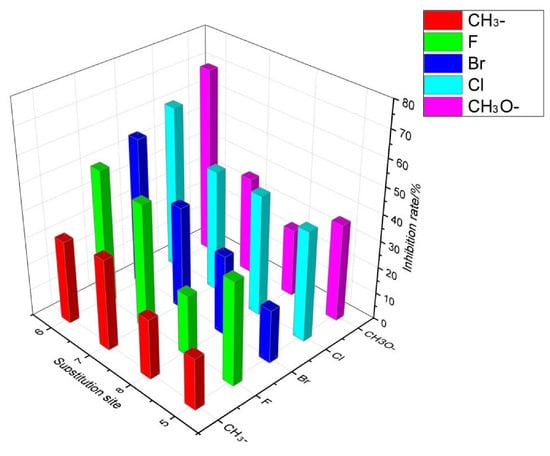
Figure 5.
Structure–activity relationship of the target compounds.
The EC50 value of 6a16 was further tested in light of its good inhibitory characteristic. The EC50 of 6a16, fluconazole, and bixafen were 9.06, 12.29, and 0.34 mg/L, respectively (Table 2), suggesting that the inhibitory activity of 6a16 against R. solani was comparable to that of fluconazole, but worse than that bixafen.
3.4. Scanning Electron Microscopy (SEM) of the Hyphae Morphology upon Treatment of R. solani with Compound 6a16
The morphology of fungi treated with 6a16 changed significantly (Figure 6). Notably, the mycelia of the control group were uniform in thickness, smooth, full on the surface, and well extended (Figure 6A). However, the mycelia appeared to fold and collapse after treatment with 9.06 mg/L and 18.12 mg/L of compound 6a16 (Figure 6C).

Figure 6.
Scanning electron micrographs of R.solani hyphae of the control group (A), hyphae exposed to 6a16 at a concentration of 9.06 mg/L (B), and hyphae exposed to 6a16 at a concentration of 18.12 mg/L (C).
4. Discussion
The nucleophilic substitution of quinazolinone can result in N- and O-substitutions [31,32]. In this study, the quinazolinones 5a1-a32 were treated with chlorinated intermediate 4 in the presence of potassium hydroxide in DMF (Scheme 3). Of note, substituting quinazolin-4-ones 5a1-a32 led to the exclusive formation of N-substituted quinazolines, with no detection of O-substituted isomers. The single-crystal X-ray diffraction of compound 6a11 further showed that the target compound was an N-substituted quinazoline. Figure 4 shows the crystal structure of 6a11, whose deposition number is CCDC 2218016.
Hybrid molecules are defined as chemical entities with two or more structural domains having different biological functions and dual activity, indicating that a hybrid molecule acts as two distinct pharmacophores [24]. Hybrid molecules could explore new lead compounds. Highly selective inhibitors of human α-1,3-Fucosyltransferase and acetylcholine esterase (AChE) were produced by this strategy [33,34]. In present paper, a quinazolinone structural unit and a pyrazole-containing active fragment were hybridized to create highly reactive molecules. We also obtained hybrid 6a16 with good antifungal activity. Therefore, molecular hybridization, based on the conjugation of quinazolinone to pyrazolecarbamide, is a useful approach for designing high antifungal candidates.
5. Conclusions
In this study, 32 novel pyrazolecarbamide derivatives bearing quinazolinone scaffolds were successfully designed, synthesized, and characterized in detail using 1H-NMR, 13C-NMR, and HRMS. The preliminary results of fungicidal bioassays revealed that some of the target compounds exhibited certain inhibitory activities against R. solani. Notably, compared with the commercial fungicide fluconazole, compound 6a16 exhibited excellent antifungal activities against R. solani by affecting the mycelial morphology. The results of this study collectively suggest that 6a16 is a lead compound against R. solani and should be further explored to enhance its utility and application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb44110380/s1.
Author Contributions
Z.L. and W.Y. conceived and designed the paper. Z.L., H.L., X.B. and X.G. contributed to the synthesis, purification, and characterization of all compounds. J.Y. and Q.P. performed the biological activity research. Z.L. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number (31860517), and by the China Postdoctoral Science Foundation, grant number (2017M623069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and supplementary material.
Acknowledgments
We deeply thank Dandan Xie for his help in elucidating the single crystal structure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siddiqui, Z.A.; Hashmi, A.; Khan, M.R.; Parveen, A. Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int.J. Veg. Sci. 2020, 26, 547–557. [Google Scholar] [CrossRef]
- Rao, T.B.; Chopperla, R.; Methre, R.; Punniakotti, E.; Venkatesh, V.; Sailaja, B.; Reddy, M.R.; Yugander, A.; Laha, G.S.; Madhav, M.S. Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol. Biol. 2019, 100, 59–71. [Google Scholar] [PubMed]
- Tan, W.; Zhang, W.; Ou, Z.; Li, C.; Zhou, G.; Wang, Z.; Yin, L. Analyses of the temporal development and yield losses due to sheath blight of rice (Rhizoctonia solani AG1. 1a). Agric. Sci. China 2007, 6, 1074–1081. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Raju, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Groth, D.E.; Miller, M.E.; Sudini, H.; Du, B.H. Evaluation of commercially available PGPR for control of rice sheath blight caused by Rhizoctonia solani. J. Pure Appl. Microbiol. 2009, 3, 485–488. [Google Scholar]
- Martins, M.A.; Frizzo, C.P.; Moreira, D.N.; Buriol, L.; Machado, P. Solvent-free heterocyclic synthesis. Chem. Rev. 2009, 109, 4140–4182. [Google Scholar]
- Ranjith, R. The chemistry and biological significance of imidazole, benzimidazole, benzoxazole, tetrazole and quinazolinone nucleus. J. Chem. Pharm. Res. 2016, 8, 505–526. [Google Scholar]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorg. Med. Chem. 2021, 50, 116476. [Google Scholar]
- Harp, T.L.; Godwin, J.R.; Scalliet, G.; Walter, H.; Stalker, A.D.; Bartlett, D.W.; Ranner, D.J. Isopyrazam, a new generation cereal fungicide. Asp. Appl. Biol. 2011, 106, 113–120. [Google Scholar]
- Ishii, H.; Zhen, F.; Hu, M.; Li, X.; Schnabel, G. Efficacy of SDHI fungicides, including benzovindiflupyr, against Colletotrichum species. Pest Manag. Sci. 2016, 72, 1844–1853. [Google Scholar] [CrossRef]
- Zeun, R.; Scalliet, G.; Oostendorp, M. Biological activity of sedaxane-a novel broad-spectrum fungicide for seed treatment. Pest Manag. Sci. 2013, 69, 527–534. [Google Scholar]
- Kocienski, P. Synthesis of Bixafen. Synfacts 2012, 8, 1283. [Google Scholar]
- Devendar, P.; Qu, R.; Kang, W.; He, B.; Yang, G. Palladium-catalyzed cross-coupling reactions: A powerful tool for the synthesis of agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. [Google Scholar] [PubMed]
- Srivastava, S.; Srivastava, S. Biological activity of Quinazoline: A review. Int. J. Pharma Sci. Res. 2015, 6, 1206–1213. [Google Scholar]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J.; Zhang, F.; Yao, Y. One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg. Med. Chem. Lett. 2016, 26, 2273–2277. [Google Scholar] [CrossRef]
- Peng, J.; Yin, X.; Li, H.; Ma, K.; Zhang, Z.; Zhou, R.; Wang, Y.; Hu, G.; Liu, Y. Design, Synthesis, and Structure–Activity Relationship of Quinazolinone Derivatives as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4 (3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Zahedifard, M.; Faraj, F.L.; Paydar, M.; Looi, C.Y.; Hasandarvish, P.; Hajrezaie, M.; Kamalidehghan, B.; Majid, N.A.; Khalifa, S.A.; Ali, H.M.; et al. Synthesis of apoptotic new quinazolinone-based compound and identification of its underlying mitochondrial signalling pathway in breast cancer cells. Curr. Pharm. Design 2015, 21, 3417–3426. [Google Scholar]
- Xie, D.; Shi, J.; Zhang, A.; Lei, Z.; Zu, G.; Fu, Y.; Gan, X.; Yin, L.; Song, B.; Hu, D. Syntheses, antiviral activities and induced resistance mechanisms of novel quinazoline derivatives containing a dithioacetal moiety. Bioorg. Chem. 2018, 80, 433–443. [Google Scholar]
- Wang, Z.; Wang, M.; Yao, X.; Li, Y.; Tan, J.; Wang, L.; Qiao, W.; Geng, Y.; Liu, Y.; Wang, Q. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar]
- Perry, C.M.; Whittington, R.; McTavish, D. Fluconazole. Drugs 1995, 49, 984–1006. [Google Scholar]
- Zervos, M.; Meunier, F. Fluconazole (Diflucan®): A review. Int. J. Antimicrob. Agents 1993, 3, 147–170. [Google Scholar]
- Pasqualotto, A.C.; Thiele, K.O.; Goldani, L.Z. Novel triazole antifungal drugs: Focus on isavuconazole, ravuconazole and albaconazole. Curr. Opin. Investig. Drugs 2010, 11, 165–174. [Google Scholar]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar]
- Liu, X.; Zhao, W.; Shen, Z.; Xing, J.; Xu, T.; Peng, W. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017, 125, 881–889. [Google Scholar] [PubMed]
- Orfi, L.; Wáczek, F.; Pató, J.; Varga, I.; Hegymegi-Barakonyi, B.; Houghten, R.A.; Ker, G. Improved, high yield synthesis of 3H-quinazolin-4-ones, the key intermediates of recently developed drugs. Curr. Med. Chem. 2004, 11, 2549–2553. [Google Scholar] [PubMed]
- Lei, Z.; Yao, J.; Liu, H.; Ma, C.; Yang, W. Synthesis and Bioactivity of Novel Sulfonate Scaffold-Containing Pyrazolecarbamide Derivatives as Antifungal and Antiviral Agents. Front. Chem. 2022, 10, 928842. [Google Scholar]
- Liu, D.; Luo, L.; Wang, Z.; Ma, X.; Gan, X. Design, Synthesis and Antifungal/Nematicidal Activity of Novel 1,2,4-Oxadiazole Derivatives Containing Amide Fragments. Int. J. Mol. Sci. 2022, 23, 1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, W.; Li, B.; Wu, G.; Ibrahim, M.; Tao, Z.; Wang, Y.; Xie, G.; Li, H.; Sun, G. Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol. Lett. 2012, 34, 2291–2298. [Google Scholar] [CrossRef]
- Plodpai, P.; Chuenchitt, S.; Petcharat, V.; Chakthong, S.; Voravuthikunchai, S.P. Anti-Rhizoctonia solani activity by Desmos chinensis extracts and its mechanism of action. Crop Prot. 2013, 43, 65–71. [Google Scholar]
- Nouira, I.; Kostakis, I.K.; Dubouilh, C.; Chosson, E.; Iannelli, M.; Besson, T. Decomposition of formamide assisted by microwaves, a tool for synthesis of nitrogen-containing heterocycles. Tetrahedron Lett. 2008, 49, 7033–7036. [Google Scholar] [CrossRef]
- Ouahrouch, A.; Taourirte, M.; Engels, J.W.; Benjelloun, S.; Lazrek, H.B. Synthesis of new 1, 2, 3-triazol-4-yl-quinazoline nucleoside and acyclonucleoside analogues. Molecules 2014, 19, 3638–3653. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [PubMed]
- Lee, L.V.; Mitchell, M.L.; Huang, S.; Fokin, V.V.; Sharpless, K.B.; Wong, C. A Potent and Highly Selective Inhibitor of Human α-1,3-Fucosyltransferase via Click Chemistry. J. Am. Chem. Soc. 2003, 125, 9588–9589. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).