Development of Duplex LAMP Technique for Detection of Porcine Epidemic Diarrhea Virus (PEDV) and Porcine Circovirus Type 2 (PCV 2)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Nucleic Acid Extraction
2.2. Design of Probes and Primers
2.3. PCR-AGE
2.4. LAMP-AGE
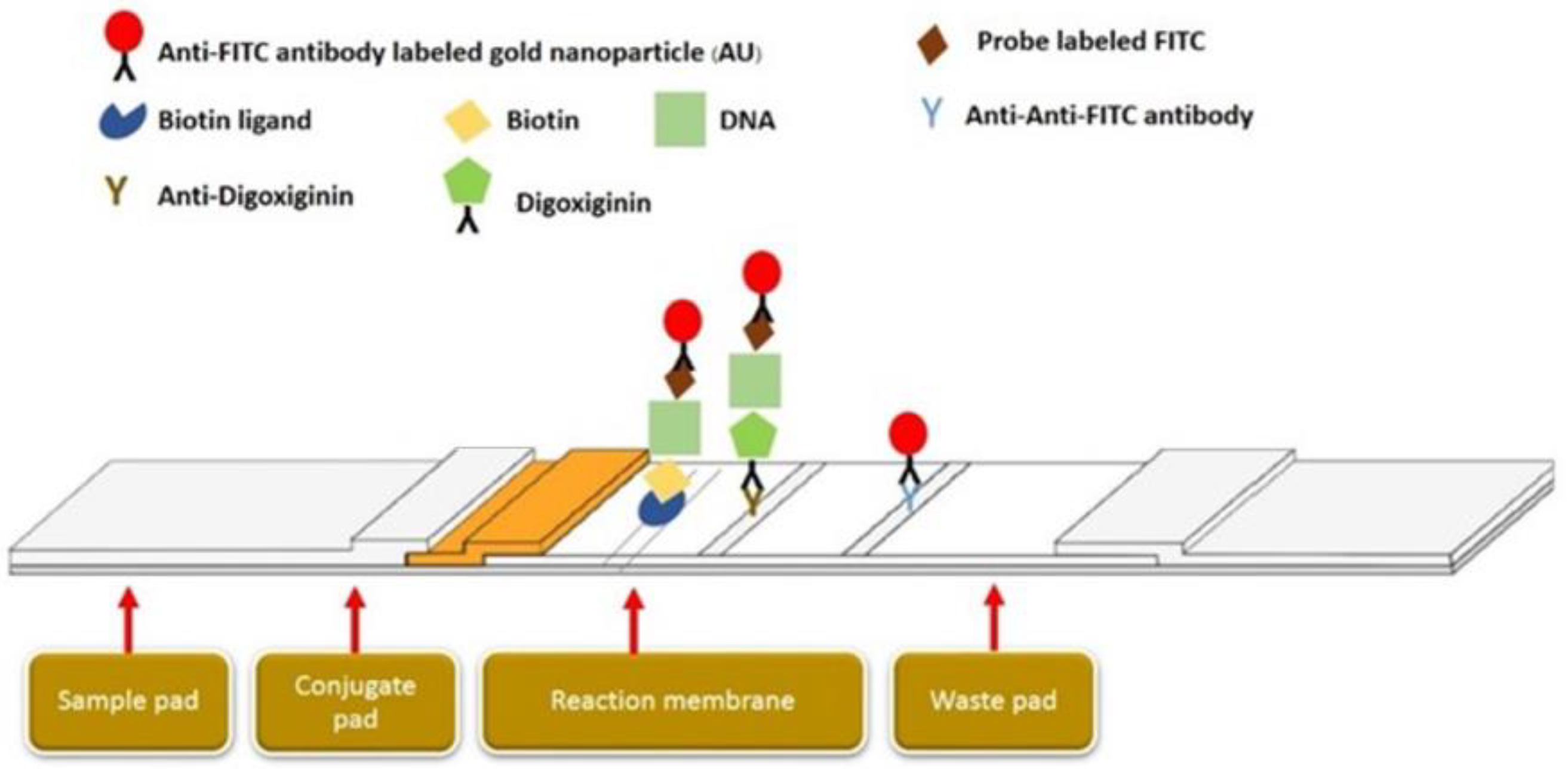
2.5. Duplex LAMP-LFD
2.6. LOD and Analytical Specificity Tests
2.7. Swine Clinical Specimens
3. Results
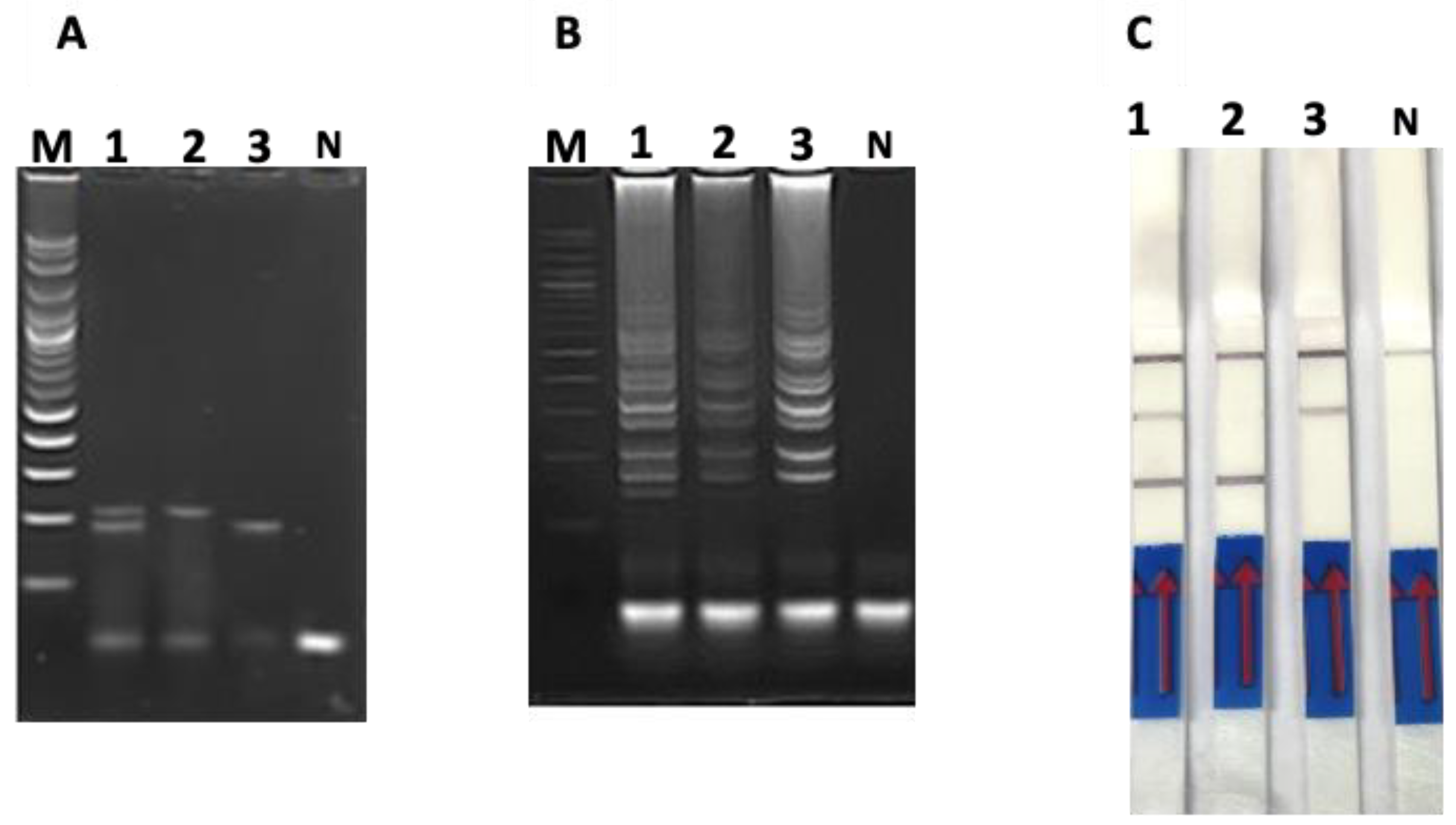
3.1. Optimization of PCR-Based AGE, LAMP-Based AGE and Duplex LAMP-LFD
3.2. LODs of PCR-Based and LAMP-Based Assays
3.3. The Analytical Specificity Tests of PCR-Based and LAMP-Based Assays
3.4. Application on Clinical Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Calsamiglia, M.; Segales, J.; Quintana, J.; Rosell, C.; Domingo, M. Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 2002, 40, 1848–1850. [Google Scholar] [CrossRef] [Green Version]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef]
- Li, R.; Tian, X.; Pang, J.; Li, L.; Yuan, J.; Tian, Z.; Wang, Z. Point-of-Care Tests for Rapid Detection of Porcine Epidemic Diarrhea Virus: A Systematic Review and Meta-Analysis. Viruses 2022, 14, 1355. [Google Scholar] [CrossRef]
- Rose, N.; Opriessnig, T.; Grasland, B.; Jestin, A. Epidemiology and transmission of porcine circovirus type 2 (PCV2). Virus Res. 2012, 164, 78–89. [Google Scholar] [CrossRef]
- Allan, G.M.; McNeilly, F.; Kennedy, S.; Daft, B.; Clarke, E.G.; Ellis, J.A.; Haines, D.M.; Meehan, B.M.; Adair, B.M. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Invest. 1998, 10, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.; Hassard, L.; Clark, E.; Harding, J.; Allan, G.; Willson, P.; Strokappe, J.; Martin, K.; McNeilly, F.; Meehan, B.; et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 1998, 39, 44–51. [Google Scholar]
- Madson, D.M.; Opriessnig, T. Effect of porcine circovirus type 2 (PCV2) infection on reproduction: Disease, vertical transmission, diagnostics and vaccination. Anim. Health Res. Rev. 2011, 12, 47–65. [Google Scholar] [CrossRef]
- Patterson, A.R.; Opriessnig, T. Epidemiology and horizontal transmission of porcine circovirus type 2 (PCV2). Anim. Health Res. Rev. 2010, 11, 217–234. [Google Scholar] [CrossRef]
- Franzo, G.; Segales, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segales, J.; Varsani, A.; Ictv Report, C. ICTV Virus Taxonomy Profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997–1998. [Google Scholar] [CrossRef]
- Franzo, G.; Segales, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [Green Version]
- Rudova, N.; Buttler, J.; Kovalenko, G.; Sushko, M.; Bolotin, V.; Muzykina, L.; Zinenko, O.; Stegniy, B.; Dunaiev, Y.; Sytiuk, M.; et al. Genetic Diversity of Porcine Circovirus 2 in Wild Boar and Domestic Pigs in Ukraine. Viruses 2022, 14, 924. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Sen, A.; Sharma, I. Development of cost-effective quantitative PCR method for parallel detection of porcine circovirus2 and porcine parvovirus in perspective of North-eastern India. Trop. Anim. Health Prod. 2021, 53, 177. [Google Scholar] [CrossRef]
- Brunborg, I.M.; Moldal, T.; Jonassen, C.M. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods 2004, 122, 171–178. [Google Scholar] [CrossRef]
- Kim, J.; Chae, C. Double in situ hybridization for simultaneous detection and differentiation of porcine circovirus 1 and 2 in pigs with postweaning multisystemic wasting syndrome. Vet. J. 2002, 164, 247–253. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, L.; Xue, Q.H.; Ning, Y.B.; Zhang, Z.G. Development of a loop-mediated isothermal amplification assay for porcine circovirus type 2. Virol. Sin. 2011, 26, 214–220. [Google Scholar] [CrossRef]
- Racine, S.; Kheyar, A.; Gagnon, C.A.; Charbonneau, B.; Dea, S. Eucaryotic expression of the nucleocapsid protein gene of porcine circovirus type 2 and use of the protein in an indirect immunofluorescence assay for serological diagnosis of postweaning multisystemic wasting syndrome in pigs. Clin. Diagn. Lab. Immunol. 2004, 11, 736–741. [Google Scholar] [CrossRef]
- Segales, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Guo, H.C.; Sun, D.H.; Yin, S.H.; Shang, Y.J.; Cai, X.P.; Liu, X.T. Development and validation of an ELISA using a protein encoded by ORF2 antigenic domain of porcine circovirus type 2. Virol. J. 2010, 7, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczotka, A.; Stadejek, T.; Pejsak, Z. A comparison of immunohistochemistry and in situ hybridization for the detection of porcine circovirus type 2 in pigs. Pol. J. Vet. Sci. 2011, 14, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Ha, Y.; Ha, S.K.; Kim, J.; Choi, C.; Park, H.K.; Kim, S.H.; Chae, C. Identification of porcine circovirus type 2 in retrospective cases of pigs naturally infected with porcine epidemic diarrhoea virus. Vet. J. 2006, 171, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Kwon, H.J.; Kim, I.H.; Hong, S.M.; Seong, W.J.; Jang, J.W.; Kim, J.H. Multiplex nested RT-PCR for detecting avian influenza virus, infectious bronchitis virus and Newcastle disease virus. J. Virol. Methods 2013, 188, 41–46. [Google Scholar] [CrossRef]
- Bigault, L.; Brown, P.; Bernard, C.; Blanchard, Y.; Grasland, B. Porcine epidemic diarrhea virus: Viral RNA detection and quantification using a validated one-step real time RT-PCR. J. Virol. Methods 2020, 283, 113906. [Google Scholar] [CrossRef]
- Cao, W.W.; He, D.S.; Chen, Z.J.; Zuo, Y.Z.; Chen, X.; Chang, Y.L.; Zhang, Z.G.; Ye, L.; Shi, L. Development of a droplet digital PCR for detection and quantification of porcine epidemic diarrhea virus. J. Vet. Diagn. Invest. 2020, 32, 572–576. [Google Scholar] [CrossRef]
- Ding, G.; Fu, Y.; Li, B.; Chen, J.; Wang, J.; Yin, B.; Sha, W.; Liu, G. Development of a multiplex RT-PCR for the detection of major diarrhoeal viruses in pig herds in China. Transbound. Emerg. Dis. 2020, 67, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Kim, S.; Song, J.; Kim, S. Highly sensitive and rapid detection of porcine circovirus 2 by avidin-biotin complex based lateral flow assay coupled to isothermal amplification. Anal. Methods 2021, 13, 4429–4436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, H.R.; Kim, D.Y.; Kim, J.M.; Kwon, N.Y.; Park, J.H.; Park, J.Y.; Kim, S.H.; Lee, K.K.; Lee, C.; et al. A simple colorimetric detection of porcine epidemic diarrhea virus by reverse transcription loop-mediated isothermal amplification assay using hydroxynaphthol blue metal indicator. J. Virol. Methods 2021, 298, 114289. [Google Scholar] [CrossRef]
- Lei, L.; Liao, F.; Tan, L.; Duan, D.; Zhan, Y.; Wang, N.; Wang, Y.; Peng, X.; Wang, K.; Huang, X.; et al. LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2. Animals 2022, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, J.; Li, X.; Qiao, D.; Wang, Z.; Wu, X.; Du, Q.; Tong, D.; Huang, Y. Establishment of method for dual simultaneous detection of PEDV and TGEV by combination of magnetic micro-particles and nanoparticles. J. Infect. Chemother. 2020, 26, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lu, J.; Wang, N.; He, W.T.; Zhang, L.; Zhao, W.; Su, S. Development of a TaqMan-probe-based multiplex real-time PCR for the simultaneous detection of emerging and reemerging swine coronaviruses. Virulence 2020, 11, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, T.; Zhang, G.; Cao, J.; Jin, Y.; Xing, G.; Liao, M.; Zhou, J. Development of a loop-mediated isothermal amplification method to rapidly detect porcine circovirus genotypes 2a and 2b. Virol. J. 2012, 9, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, G.; Niu, J.; Zhou, X.; Xie, Y.; Chen, Z.; Li, G.; Chen, R.; He, D. Use of dual priming oligonucleotide system-based multiplex RT-PCR assay to detect five diarrhea viruses in pig herds in South China. AMB Express 2021, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Victorious, A.; Zhang, Z.; Chang, D.; Maclachlan, R.; Pandey, R.; Xia, J.; Gu, J.; Hoare, T.; Soleymani, L.; Li, Y. A DNA Barcode-Based Aptasensor Enables Rapid Testing of Porcine Epidemic Diarrhea Viruses in Swine Saliva Using Electrochemical Readout. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204252. [Google Scholar]
- Yuan, X.; Lv, J.; Lin, X.; Zhang, C.; Deng, J.; Wang, C.; Fan, X.; Wang, Y.; Xu, H.; Wu, S. Multiplex detection of six swine viruses on an integrated centrifugal disk using loop-mediated isothermal amplification. J. Vet. Diagn. Invest. 2019, 31, 415–425. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Li, Q.; Wu, M.; Lei, L.; Pan, Z. A multiplex RT-PCR assay for rapid and simultaneous detection of four RNA viruses in swine. J. Virol. Methods 2019, 269, 38–42. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Y.; Fang, X.; Liu, Y.; Du, M.; Lu, X.; Li, Q.; Sun, Y.; Ma, J.; Lan, T. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine. Anal. Chim. Acta 2020, 1125, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, S.; Shi, J.; Wu, J.; Yuan, X.; Cong, X.; Xu, S.; Wu, X.; Li, J.; Wang, J. Loop-mediated isothermal amplification for detection of porcine circovirus type 2. Virol. J. 2011, 8, 497. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequence (5′ –> 3′) | 5′ Conjugated |
|---|---|---|
| PCV2_F3 | GCTGGCTGAACTTTTGAAAG | - |
| PCV2_B3 | AGCCAGCCATAAAAGTCA | - |
| PCV2_FIP | GCTTTTACCACACCCAGGTGTTTTTGAGCGGGAAAATGCAGAA | Biotin |
| PCV2_BIP | GACCCGGAAACCACATACTGGTTTTTTCAATAACAACCACCACTTCTTCAC | - |
| PEDV-F3 | CTTGAAGGTGTCACGGAC | - |
| PEDV-B3 | CAGAATAAACAGCACCACTAG | - |
| PEDV-FIP | ATGATACCCTCACCTTTAAAGCCTTTTTTTTTATGACTCTGGATGTGTG | Digoxigenin |
| PEDV-BIP | CCTTACAAATTCTAGCTTTTTGGCATTTTTACATTCTTAAAGGCTAACAACT | - |
| Probe_PCV2_FITC | TGCAAAATTAGCCCATT | FITC |
| Probe_PEDV_FITC | GAATCAGATGTGTAATAAAC | FITC |
| PCR-Based AGE | |||
|---|---|---|---|
| Positive | Negative | ||
| Duplex LAMP-LFD | Positive | True positive (TP) | False positive (FP) |
| Negative | False negative (FN) | True negative (TN) | |
| Diagnosis Method | True Pedv Positive | True PCV2 Positive | True Negative | Diagnostic Sensitivity (%) | Diagnostic Specificity (%) | Accuracy (%) | Total Time of Detection |
|---|---|---|---|---|---|---|---|
| PCR-based AGE | 16 | 14 | 20 | 100 | 100 | 100 | 2.5 h |
| LAMP-based AGE | 16 | 14 | 20 | 100 | 100 | 100 | 2 h |
| Duplex LAMP-LFD | 16 | 14 | 20 | 100 | 100 | 100 | 1.5 h |
| Viruses | Methods | Samples | Limit of Detection | Analytical Specificity | % Diagnostic Sensitivity | % Diagnostic Specificity | % Accuracy | % Sensitivity References |
|---|---|---|---|---|---|---|---|---|
| PEDV, PCV2 | Duplex-LAMP-LFD | Clinical specimens | 0.1 ng/μL 0.246 ng/µL | No cross-reaction with FMDV, ADV, TGEV, PRRSV (EU strain and US strain), CSFV, SIV. | 100% | 100% | 100% | This study |
| PEDV, TGEV, PRV-A, PKV, PsaV, PDCoV | Multiplex RT-PCR | Feces | 100–101 ng cDNA of each virus | No cross-reaction with any other major viruses in swine. | N/A* | N/A* | N/A* | [30] |
| PEDV, PDCoV, PToV, SADS-CoV | Taq Man-probe-based multiplex real-time PCR | Feces | 1 × 102 copies/μL | No cross-reaction with TGEV, PoRV, PSV, PTV, CSFV, PKV. | N/A* | N/A* | N/A* | [35] |
| PEDV, TGEV | Dual ultrasensitive nanoparticle DNA probe-based PCR assay (dual UNDP-PCR) | Feces | 25 copies/g | No cross-reaction with PPV, PRV, CSFV, PCV2, PRRSV. | N/A* | N/A* | N/A* | [34] |
| PEDV | RT-LAMP combined with hydroxynaphthol blue metal indicator | Feces, small intestine | 50 RNA copies per reaction | No cross-reaction with TGEV, PDCoV, PRV, type 1 and 2 PRRSVs, CSFV, PCV2, PPV, SIV. | N/A* | N/A* | N/A* | [32] |
| PEDV, PDCoV, SADS-CoV | Microfluidic-RT-LAMP chip | Feces, intestinal contents | 101 copies/μL, 102 copies/μL 102 copies/μL | No cross-reaction with CSFV, PPV, JEV, PCV2, PRRSV, PRV, SIV, FMDV, SVV, RV, TGEV. | 92.24%, 92.19% 91.23% | 100% 100% 100% | N/A* | [41] |
| PEDV | Droplet digital PCR | Small intestine, feces, serum | 0.26 copies/μL | No cross-reaction with PRV, PRRSV, PDCoV, CSFV, TGEV, PRCV, SADS-CoV, Actinobacillus pleuropneumoniae, Haemophilus parasuis, Streptococcus suis, Escherichia coli, Salmonella typhimurium, Clostridium (Clostridioides) difficile, Clostridium perfringens. | 98.6% | 100% | N/A* | [29] |
| PEDV, TGEV, CSFV, PRRSV | Multiplex RT-PCR assay | Clinical specimens | 1 × 105 copies 1 × 103 copies 1 × 103 copies 1 × 103 copies | No cross-reaction with PCV2, BVDV, RSV, H5N1. | N/A* | N/A* | N/A* | [40] |
| PEDV | One-step real-time RT-PCR | Spiked Feces, Spiked jejunum matrices | 50 copies/5 μL 100 copies/5 μl | No cross-reaction with PRCV, IBV, TCoV, PRRSV, CSFV, ASFV, SIV-H1N1, SIV-H1N2, SIV-H3N2, H3N2, PCV2. | 100% | 100% | N/A* | [28] |
| PEDV | DNA Barcode-Based Aptasensor | Saliva | 0.37 μg/mL | N/A | 83% | 100% | N/A* | [38] |
| PEDV, TGEV, PRV-A, PDCoV, SADS-CoV | Dual priming oligonucleotide system-based multiplex RT-PCR assay | Intestinal samples | 103–104 copies/μL plasmid of each virus | No cross-reaction with PRRSV, APPV, SVV, PCV3, PRCV, PTV, PPV. | 100% coincidence rate with that of the RT-PCR method in the evaluation of 181 swine intestinal samples. | [37] | ||
| PCV2 | LAMP Coupled CRISPR-Cas12a Module | Blood | 1 copy/μL | No cross-reaction with PCV1, PCV3, PEDV, CSFV, PRRSV. | 100% coincidence rate with that of the quantitative PCR (qPCR) method in the evaluation of 30 clinical blood samples. | [33] | ||
| PCV2 | LAMP-LFD | DNA | 10 fg | CSFV, Erysipelothrix rhusiopathiae, PRRSV type 1, PRRSV type 2, PCV3, SIV-H1N1, SIV-H1N2, and SIV-H3N2. | N/A* | N/A* | N/A* | [31] |
| PCV2a, PCV2b | LAMP | Clinical samples | 103 copies/reaction | No cross-reaction with PCV1, PPV, PRV, PRRSV. | 97.7% | 100% | 98.2% | [36] |
| PCV2 | LAMP-SYBR Green I Dye | Clinical samples | 1 copy | No cross-reaction with PCV, PRV and PPV. | 100% | 86.96% | 89.66% | [42] |
| PCV2, PRRSV, PRV, PPV, FMDV | Centrifugal microfluidic disk (CMFD) using LAMP | Clinical samples | 3.2 × 102 copies per reaction | No cross-reaction with TGEV, PEDV, PoRV. | 94.0% coincidence rate with PCR in the evaluation of 232 clinical samples. | [39] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areekit, S.; Tangjitrungrot, P.; Khuchareontaworn, S.; Rattanathanawan, K.; Jaratsing, P.; Yasawong, M.; Chansiri, G.; Viseshakul, N.; Chansiri, K. Development of Duplex LAMP Technique for Detection of Porcine Epidemic Diarrhea Virus (PEDV) and Porcine Circovirus Type 2 (PCV 2). Curr. Issues Mol. Biol. 2022, 44, 5427-5439. https://doi.org/10.3390/cimb44110368
Areekit S, Tangjitrungrot P, Khuchareontaworn S, Rattanathanawan K, Jaratsing P, Yasawong M, Chansiri G, Viseshakul N, Chansiri K. Development of Duplex LAMP Technique for Detection of Porcine Epidemic Diarrhea Virus (PEDV) and Porcine Circovirus Type 2 (PCV 2). Current Issues in Molecular Biology. 2022; 44(11):5427-5439. https://doi.org/10.3390/cimb44110368
Chicago/Turabian StyleAreekit, Supatra, Pongbun Tangjitrungrot, Sintawee Khuchareontaworn, Kankanit Rattanathanawan, Pornpun Jaratsing, Montri Yasawong, Gaysorn Chansiri, Nareerat Viseshakul, and Kosum Chansiri. 2022. "Development of Duplex LAMP Technique for Detection of Porcine Epidemic Diarrhea Virus (PEDV) and Porcine Circovirus Type 2 (PCV 2)" Current Issues in Molecular Biology 44, no. 11: 5427-5439. https://doi.org/10.3390/cimb44110368
APA StyleAreekit, S., Tangjitrungrot, P., Khuchareontaworn, S., Rattanathanawan, K., Jaratsing, P., Yasawong, M., Chansiri, G., Viseshakul, N., & Chansiri, K. (2022). Development of Duplex LAMP Technique for Detection of Porcine Epidemic Diarrhea Virus (PEDV) and Porcine Circovirus Type 2 (PCV 2). Current Issues in Molecular Biology, 44(11), 5427-5439. https://doi.org/10.3390/cimb44110368






