Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection and Preparation
2.2. Sperm Capacitation
2.3. Immunoprecipitation
2.4. Identification of tACE Interactome by Liquid Chromatography and Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.5. LC-MS/MS Analysis
2.6. Raw Data Conversion
2.7. Gene Ontology and Interactome Analyses
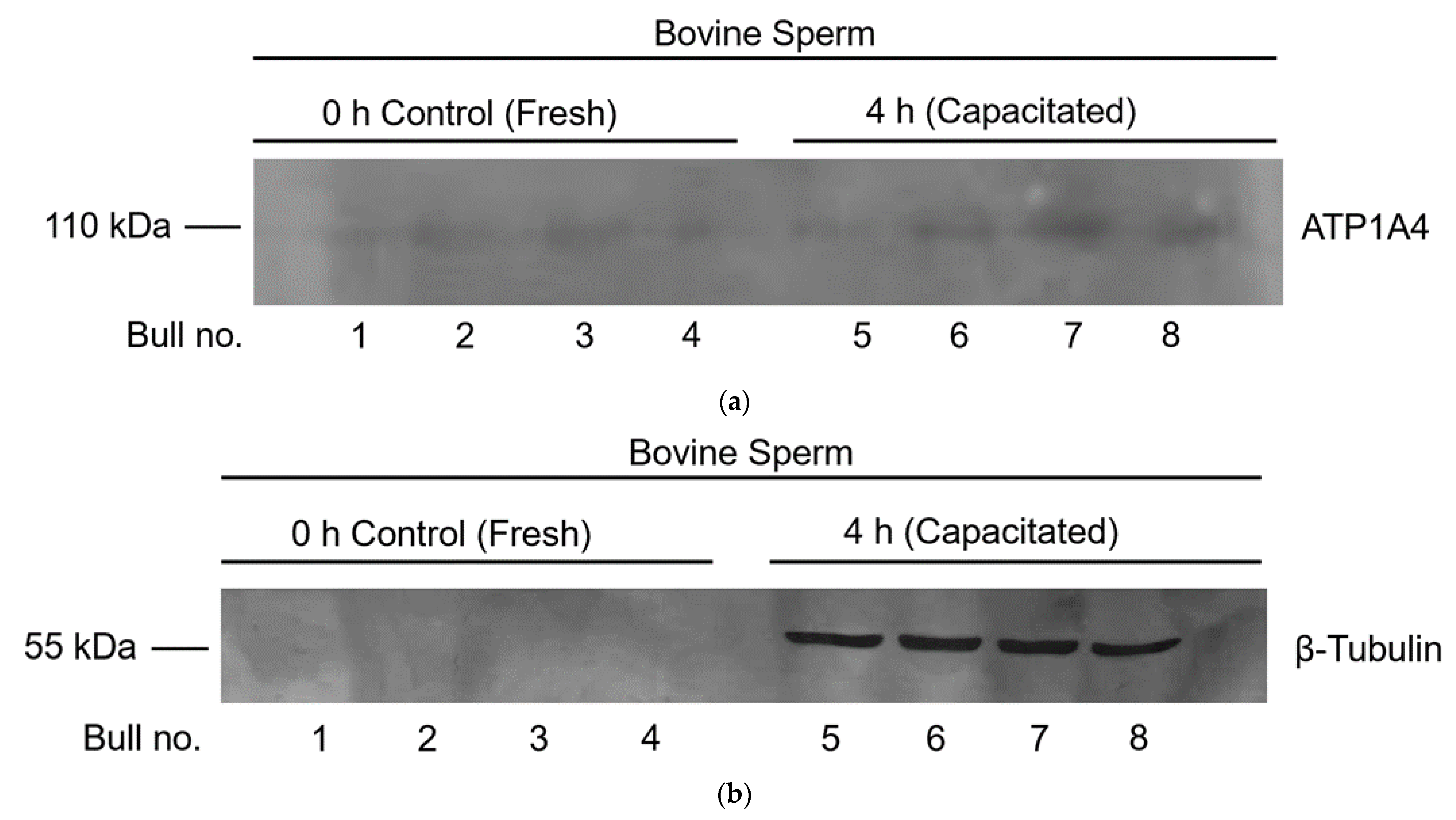
2.8. Immunodetection of ATP1A4 in the tACE Interactome
2.9. Immunocytochemistry for Co-Localization of β-Tubulin and tACE
2.10. Statistical Analysis
3. Results
3.1. Mass Spectrometry for Characterization of the tACE Interactome
3.2. Gene Ontological Characterization of the tACE Interactome
3.3. Interactive Network of Constituents of the tACE Interactome
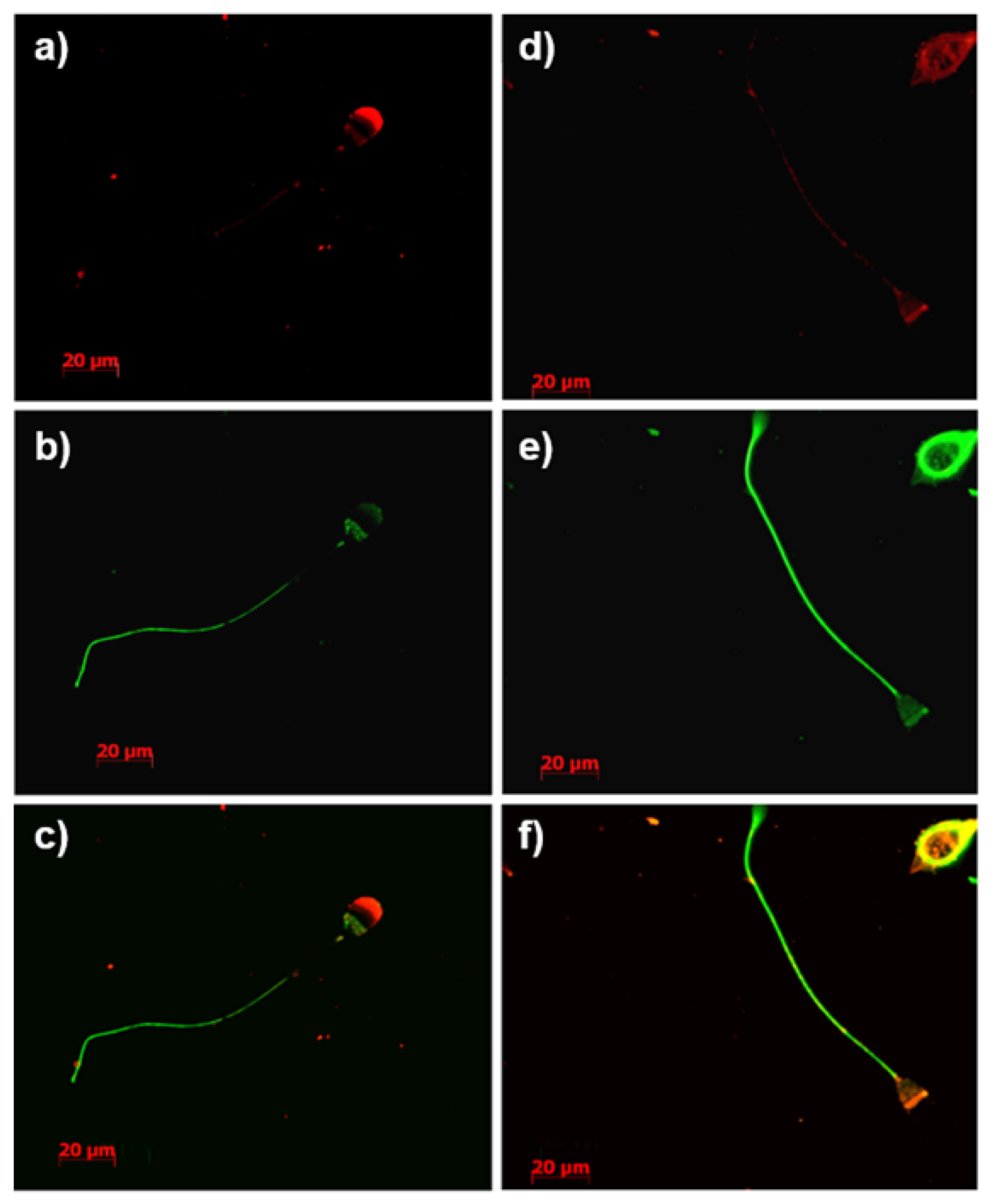
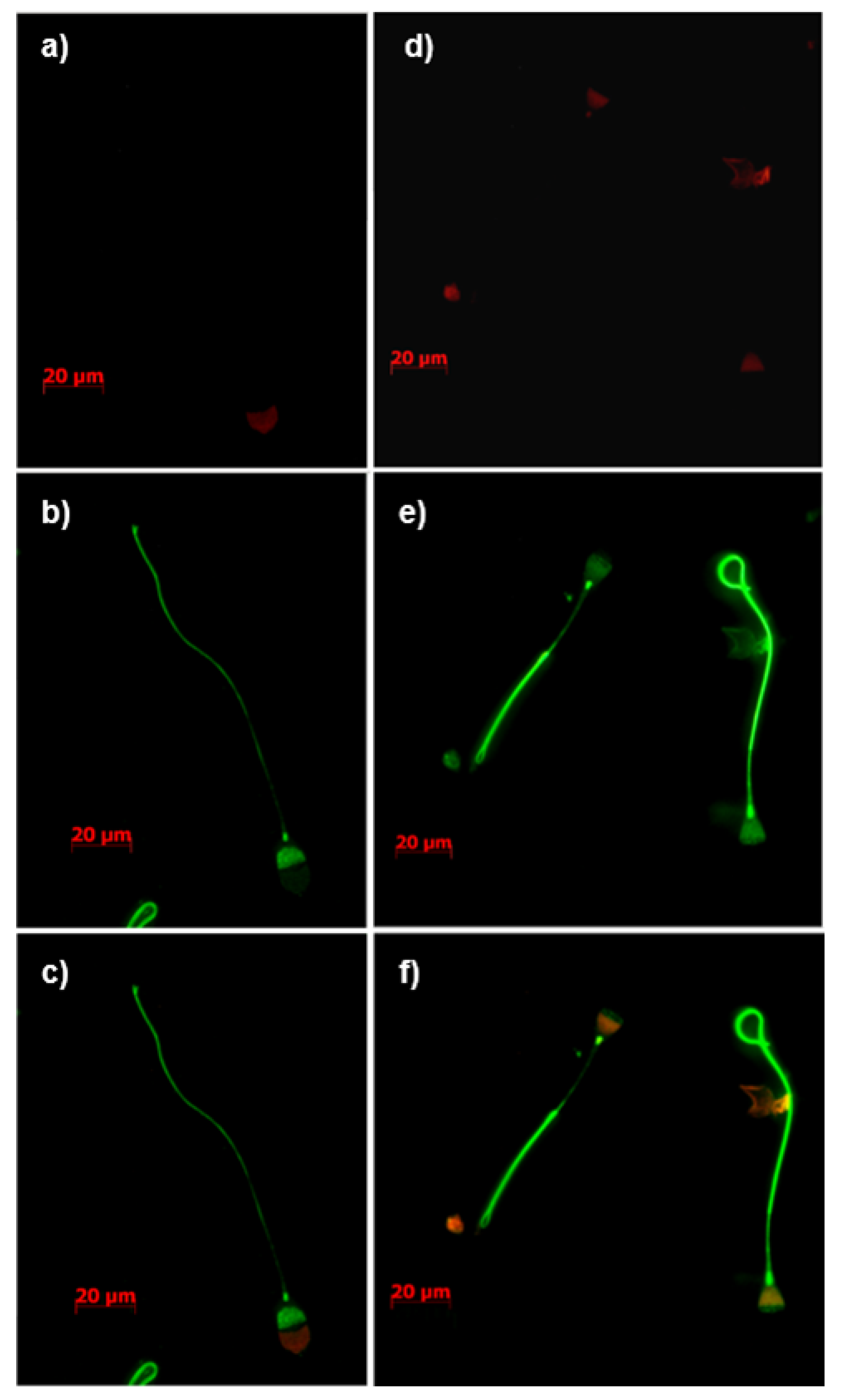
3.4. Immunolocalization of Constituents of the tACE Interactome
3.5. Immunodetection of Identified Constituents of the tACE Interactome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef]
- Senger, P.L. Pathways to Pregnancy & Parturition, 3rd ed.; Current Conceptions, Inc.: Redmond, OR, USA, 2012; pp. 255–260. [Google Scholar]
- Yanagimachi, R.; Chang, M. Fertilization of hamster eggs in vitro. Nature 1963, 200, 281–282. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Huang, S.; Zhou, T.; Guo, Y.; Wang, G.; Guo, X.; Zhou, Z.; Sha, J. Quantitative phosphoproteomics analysis reveals a key role of insulin growth factor 1 receptor (IGF1R) tyrosine kinase in human sperm capacitation. Mol. Cell. Proteom. 2015, 14, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Takeo, T.; Irie, T.; Nakagata, N. Fertility of cold-stored mouse sperm is recovered by promoting acrosome reaction and hyperactivation after cholesterol efflux by methyl-beta-cyclodextrin. Biol. Reprod. 2017, 96, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Galantino-Homer, H.; Ning, X.; Moore, G.D.; Valenzuela, J.P.; Jorgez, C.J.; Alvarez, J.G.; Kopf, G.S. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 1999, 274, 3235–3242. [Google Scholar] [CrossRef]
- Rahman, M.S.; Lee, J.; Kwon, W.; Pang, M. Sperm proteomics: Road to male fertility and contraception. Int. J. Endocrinol. 2013, 2013, 360986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and Protein Synthesis in Mitochondria Enhance the Duration of High-Speed Linear Motility in Boar Sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Content of testis-specific isoform of Na/K-ATPase (ATP1A4) is increased during bovine sperm capacitation through translation in mitochondrial ribosomes. Cell Tissue Res. 2017, 368, 187–200. [Google Scholar] [CrossRef]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef]
- Fuchs, S.; Frenzel, K.; Hubert, C.; Lyng, R.; Muller, L.; Michaud, A.; Xiao, H.D.; Adams, J.W.; Capecchi, M.R.; Corvol, P.; et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nat. Med. 2005, 11, 1140–1142. [Google Scholar] [CrossRef]
- Kondoh, G.; Tojo, H.; Nakatani, Y.; Komazawa, N.; Murata, C.; Yamagata, K.; Maeda, Y.; Kinoshita, T.; Okabe, M.; Taguchi, R.; et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 2005, 11, 160–166. [Google Scholar] [CrossRef]
- Ojaghi, M.; Kastelic, J.; Thundathil, J.C. Testis-specific isoform of angiotensin-converting enzyme (tACE) is involved in the regulation of bovine sperm capacitation. Mol. Reprod. Dev. 2017, 84, 376–388. [Google Scholar] [CrossRef]
- Ojaghi, M.; Johnson, C.; Rizzoto, G.; Kastelic, J.; Thundathil, J. Content and activity of the testis-specific isoform of angiotensin-converting enzyme are reduced in frozen-thawed bull spermatozoa. Reprod. Fertil. Dev. 2018, 30, 1575–1583. [Google Scholar] [CrossRef]
- Ojaghi, M.; Kastelic, J.; Thundathil, J. Testis-specific isoform of angiotensin-converting enzyme (tACE) as a candidate marker for bull fertility. Reprod. Fertil. Dev. 2018, 30, 1584–1593. [Google Scholar] [CrossRef]
- Hagaman, J.R.; Moyer, J.S.; Bachman, E.S.; Sibony, M.; Magyar, P.L.; Welch, J.E.; Smithies, O.; Krege, J.H.; O’Brien, D.A. Angiotensin-Converting Enzyme and Male Fertility. Proc. Natl. Acad. Sci. USA 1998, 95, 2552–2557. [Google Scholar] [CrossRef]
- Krege, J.; John, S.; Langenbach, L.; Hodgin, J.; Hagaman, J.; Bachman, E.; Jennette, J.; O’Brien, D.; Smithies, O. Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature 1995, 375, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.P.; Rowe, T.M.; Gomos, J.B.; Kessler, P.M.; Sen, G.C. Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J. Biol. Chem. 2000, 275, 26259–26264. [Google Scholar] [CrossRef] [PubMed]
- Hooper, N.M.; Turner, A.J. An ACE structure. Nat. Struct. Mol. Biol. 2003, 10, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Leisle, L.; Parkin, E.T.; Turner, A.J.; Hooper, N.M. Angiotensin-converting enzyme as a GPIase: A critical reevaluation. Nat. Med. 2005, 11, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Kamaruddin, M.; Kroetsch, T.; Basrur, P.K.; Hansen, P.J.; King, W.A. Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia 2004, 36, 327–334. [Google Scholar] [CrossRef]
- Newton, L.D.; Krishnakumar, S.; Menon, A.G.; Kastelic, J.P.; van der Hoorn, F.; Thundathil, J.C. Na+/K+ATPase regulates sperm capacitation through a mechanism involving kinases and redistribution of its testis-specific isoform. Mol. Reprod. Dev. 2010, 77, 136–148. [Google Scholar] [CrossRef]
- Castillo, J.; Bogle, O.A.; Jodar, M.; Torabi, F.; Delgado-Dueñas, D.; Estanyol, J.M.; Ballescà, J.L.; Miller, D.; Oliva, R. Proteomic Changes in Human Sperm During Sequential in vitro Capacitation and Acrosome Reaction. Front. Cell Dev. Biol. 2019, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Testis-Specific Isoform of Na/K-ATPase (ATP1A4) Interactome in Raft and Non-Raft Membrane Fractions from Capacitated Bovine Sperm. Int. J. Mol. Sci. 2019, 20, 3159. [Google Scholar] [CrossRef]
- Fardilha, M.; Esteves, S.L.; Korrodi-Gregório, L.; Vintém, A.P.; Domingues, S.C.; Rebelo, S.; Morrice, N.; Cohen, P.T.; da Cruz e Silva, O.A.B.; da Cruz e Silva, E.F. Identification of the human testis protein phosphatase 1 interactome. Biochem. Pharmacol. 2011, 82, 1403–1415. [Google Scholar] [CrossRef]
- Galantino-Homer, H.L.; Visconti, P.E.; Kopf, G.S. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3’5’-monophosphate-dependent pathway. Biol. Reprod. 1997, 56, 707–719. [Google Scholar] [CrossRef]
- Newton, L.D.; Kastelic, J.P.; Wong, B.; van der Hoorn, F.; Thundathil, J. Elevated testicular temperature modulates expression patterns of sperm proteins in Holstein bulls. Mol. Reprod. Dev. 2009, 76, 109–118. [Google Scholar] [CrossRef]
- Inoue, N.; Kasahara, T.; Ikawa, M.; Okabe, M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS ONE 2010, 5, e10301. [Google Scholar] [CrossRef] [PubMed]
- Teijeiro, J.M.; Marini, P.E. The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro-AKAP4 phosphorylation. Reproduction 2012, 143, 773–785. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Dudiki, T.; Vijayaraghavan, S.; Carr, D.W. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol. Reprod. 2013, 88, 1–10. [Google Scholar] [CrossRef]
- Rahamim Ben-Navi, L.; Almog, T.; Yao, Z.; Seger, R.; Naor, Z. A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 2016, 6, 37922. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Ritagliati, C.; Xu, X.; Gervasi, M.G.; Luque, G.M.; Baró Graf, C.; De la Vega-Beltrán, J.L.; Torres, N.; Darszon, A.; Krapf, D.; et al. Disruption of protein kinase A localization induces acrosomal exocytosis in capacitated mouse sperm. J. Biol. Chem. 2018, 293, 9435–9447. [Google Scholar] [CrossRef] [PubMed]
- Hillman, P.; Ickowicz, D.; Vizel, R.; Breitbart, H. Dissociation between AKAP3 and PKARII promotes AKAP3 degradation in sperm capacitation. PLoS ONE 2013, 8, e68873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, W.; Mandal, A.; Kim, Y.H.; Digilio, L.; Klotz, K.; Flickinger, C.J.; Herr, J.C. CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J. Androl. 2011, 13, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, W.; Kim, Y.H.; Mandal, A.; Digilio, L.; Klotz, K.; Flickinger, C.J.; Herr, J.C. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod. Biol. Endocrinol. 2010, 8, 101. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Mandal, A.; Wolkowicz, M.; Sen, B.; Westbrook, V.; Shetty, J.; Coonrod, S.; Klotz, K.; Kim, Y.; Bush, L.; et al. CABYR, a Novel Calcium-Binding Tyrosine Phosphorylation-Regulated Fibrous Sheath Protein Involved in Capacitation. Dev. Biol. 2002, 242, 236–254. [Google Scholar] [CrossRef]
- Donkor, F.F.; Mönnich, M.; Czirr, E.; Hollemann, T.; Hoyer-Fender, S. Outer dense fibre protein 2 (ODF2) is a self-interacting centrosomal protein with affinity for microtubules. J. Cell Sci. 2004, 117, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Mariappa, D.; Aladakatti, R.H.; Dasari, S.K.; Sreekumar, A.; Wolkowicz, M.; van der Hoorn, F.; Seshagiri, P.B. Inhibition of tyrosine phosphorylation of sperm flagellar proteins, outer dense fiber protein-2 and tektin-2, is associated with impaired motility during capacitation of hamster spermatozoa. Mol. Reprod. Dev. 2010, 77, 182–193. [Google Scholar] [CrossRef]
- Tarnasky, H.; Cheng, M.; Ou, Y.; Thundathil, J.C.; Oko, R.; van der Hoorn, F.A. Gene trap mutation of murine outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev. Biol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Ito, C.; Akutsu, H.; Yao, R.; Yoshida, K.; Yamatoya, K.; Mutoh, T.; Makino, T.; Aoyama, K.; Ishikawa, H.; Kunimoto, K.; et al. Odf2 haploinsufficiency causes a new type of decapitated and decaudated spermatozoa, Odf2-DDS, in mice. Sci. Rep. 2019, 9, 14249. [Google Scholar] [CrossRef]
- Travis, A.J.; Jorgez, C.J.; Merdiushev, T.; Jones, B.H.; Dess, D.M.; Diaz-Cueto, L.; Storey, B.T.; Kopf, G.S.; Moss, S.B. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J. Biol. Chem. 2001, 276, 7630–7636. [Google Scholar] [CrossRef]
- Naz, R.K.; Morte, C.; Ahmad, K.; Martinez, P. Hexokinase present in human sperm is not tyrosine phosphorylated but its antibodies affect fertilizing capacity. J. Androl. 1996, 17, 143–150. [Google Scholar] [CrossRef]
- Leyton, L.; Saling, P. 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell 1989, 57, 1123–1130. [Google Scholar] [CrossRef]
- Kalab, P.; Visconti, P.; Leclerc, P.; Kopf, G.S. p95, the major phosphotyrosine-containing protein in mouse spermatozoa, is a hexokinase with unique properties. J. Biol. Chem. 1994, 269, 3810–3817. [Google Scholar] [CrossRef]
- Visconti, P.E.; Olds-Clarke, P.; Moss, S.B.; Kalab, P.; Travis, A.J.; de las Heras, M.; Kopf, G.S. Properties and localization of a tyrosine phosphorylated form of hexokinase in mouse sperm. Mol. Reprod. Dev. 1996, 43, 82–93. [Google Scholar] [CrossRef]
- Leyton, L.; Tomes, C.; Saling, P. LL95 monoclonal antibody mimics functional effects of ZP3 on mouse sperm: Evidence that the antigen recognized is not hexokinase. Mol. Reprod. Dev. 1995, 42, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Na/K-ATPase regulates bovine sperm capacitation through raft- and non-raft-mediated signaling mechanisms. Mol. Reprod. Dev. 2017, 84, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Thundathil, J.C.; Anzar, M.; Buhr, M.M. Na+/K+ATPase as a signaling molecule during bovine sperm capacitation. Biol. Reprod. 2006, 75, 308–317. [Google Scholar] [CrossRef]
- Jimenez, T.; McDermott, J.P.; Sánchez, G.; Blanco, G. Na, K-ATPase α4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. USA 2011, 108, 644–649. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Okabe, M. The mechanism of sperm-egg interaction and the involvement of IZUMO1 in fusion. Asian J. Androl. 2011, 13, 81–87. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Curry, B.J.; Hall, S.E.; Aitken, R.J. Aldehyde Dehydrogenase Plays a Pivotal Role in the Maintenance of Stallion Sperm Motility. Biol. Reprod. 2016, 94, 133. [Google Scholar] [CrossRef]
- Koppers, A.J.; Garg, M.L.; Aitken, R.J. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic. Biol. Med. 2010, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Brunati, A.; Ragazzi, E.; Armanini, D.; Bordin, L.; Clari, G. Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum. Reprod. 2011, 26, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, M.S.; Nichols, R.J.; Molitor, T.P.; Lindgren, J.K.; Traktman, P. Mice deficient in the serine/threonine protein kinase VRK1 are infertile due to a progressive loss of spermatogonia. Biol. Reprod. 2010, 82, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Park, C.H.; Kim, W.; Ling, H.; Kang, A.; Chang, M.W.; Im, S.K.; Jeong, H.W.; Kong, Y.Y.; Kim, K.T. Vaccinia-related kinase 1 is required for the maintenance of undifferentiated spermatogonia in mouse male germ cells. PLoS ONE 2010, 5, e15254. [Google Scholar] [CrossRef]
- Kang, T.; Kim, K. Negative regulation of ERK activity by VRK3-mediated activation of VHR phosphatase. Nat. Cell Biol. 2006, 8, 863–869. [Google Scholar] [CrossRef]
- Kang, T.; Kim, K. VRK3-mediated inactivation of ERK signaling in adult and embryonic rodent tissues. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 49–58. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol. Hum. Reprod. 2002, 8, 124–135. [Google Scholar] [CrossRef]
- Thundathil, J.C.; Rajamanickam, G.D.; Kastelic, J.P.; Newton, L.D. The effects of increased testicular temperature on testis-specific isoform of Na+/K+ -ATPase in sperm and its role in spermatogenesis and sperm function. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 170–177. [Google Scholar] [CrossRef]
- Hashemitabar, M.; Sabbagh, S.; Orazizadeh, M.; Ghadiri, A.; Bahmanzadeh, M. A proteomic analysis on human sperm tail: Comparison between normozoospermia and asthenozoospermia. J. Assist. Reprod. Genet. 2015, 32, 853–863. [Google Scholar] [CrossRef]
- Agarwal, A.; Selvam, M.K.P.; Baskaran, S. Proteomic Analyses of Human Sperm Cells: Understanding the Role of Proteins and Molecular Pathways Affecting Male Reproductive Health. Int. J. Mol. Sci. 2020, 21, 1621. [Google Scholar] [CrossRef]
- Cao, X.; Cui, Y.; Zhang, X.; Lou, J.; Zhou, J.; Bei, H.; Wei, R. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod. Biol. Endocrinol. 2018, 16, 16. [Google Scholar] [CrossRef]
- Bracke, A.; Peeters, K.; Punjabi, U.; Hoogewijs, D.; Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 2018, 36, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Pĕknicová, J.; Pexidrova, M.; Kubatova, A.; Koubek, P.; Tepla, O.; Sulimenko, T.; Draber, P. Expression of beta-tubulin epitope in human sperm with pathological spermiogram. Fertil. Steril. 2007, 88, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Dvoráková, K.; Moore, H.D.; Sebková, N.; Palecek, J. Cytoskeleton localization in the sperm head prior to fertilization. Reproduction 2005, 130, 61–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dráber, P.; Dráberová, E.; Viklický, V. Immunostaining of human spermatozoa with tubulin domain-specific monoclonal antibodies. Recognition of a unique beta-tubulin epitope in the sperm head. Histochemistry 1991, 95, 519–524. [Google Scholar] [CrossRef]
- Pĕknicová, J.; Kubátová, A.; Sulimenko, V.; Dráberová, E.; Viklický, V.; Hozák, P.; Dráber, P. Differential subcellular distribution of tubulin epitopes in boar spermatozoa: Recognition of class III beta-tubulin epitope in sperm tail. Biol. Reprod. 2001, 65, 672–679. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Bajpai, M.; Carr, D.W. Identification and characterization of RHOA-interacting proteins in bovine spermatozoa. Biol. Reprod. 2008, 78, 184–192. [Google Scholar] [CrossRef]
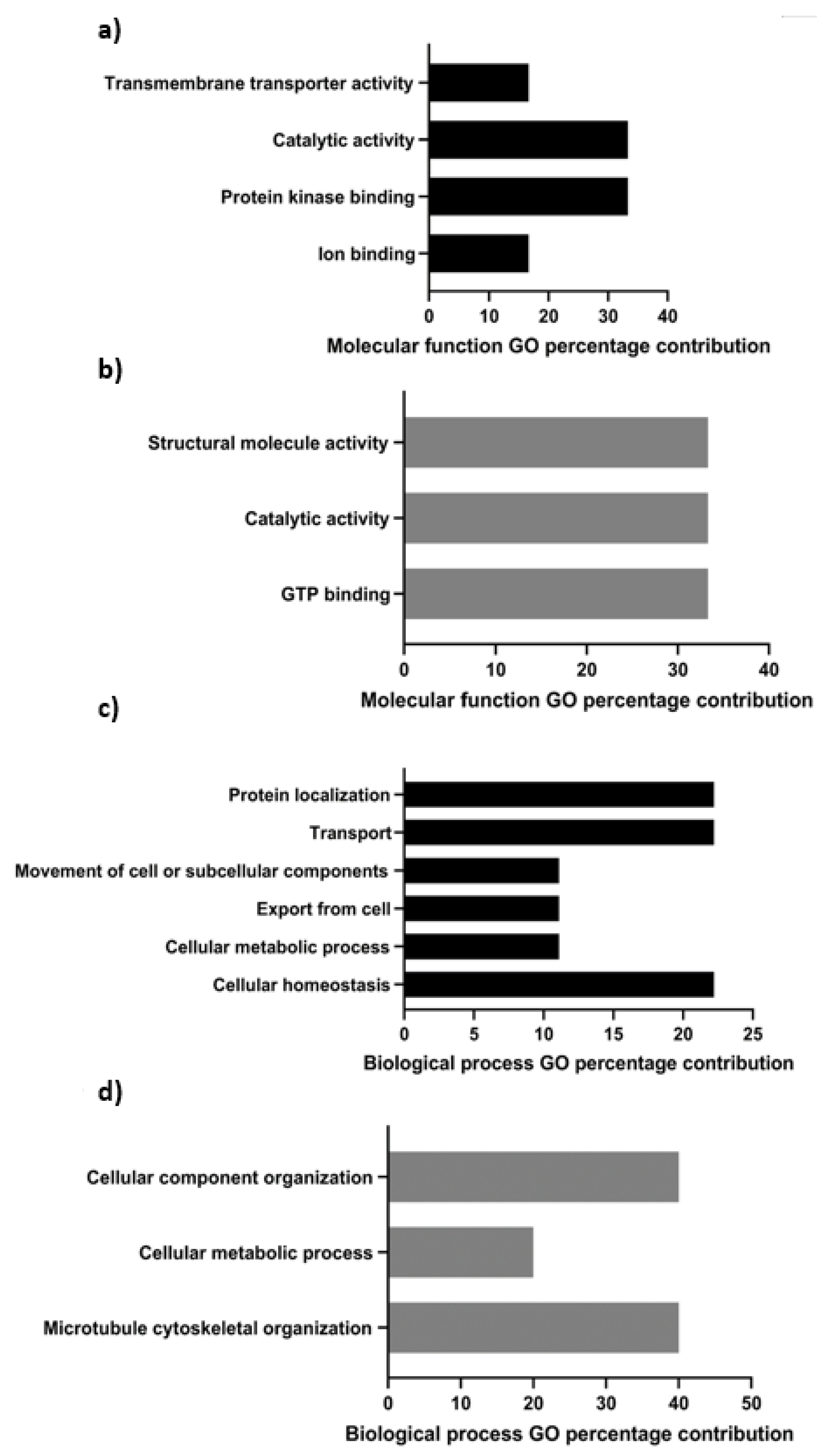

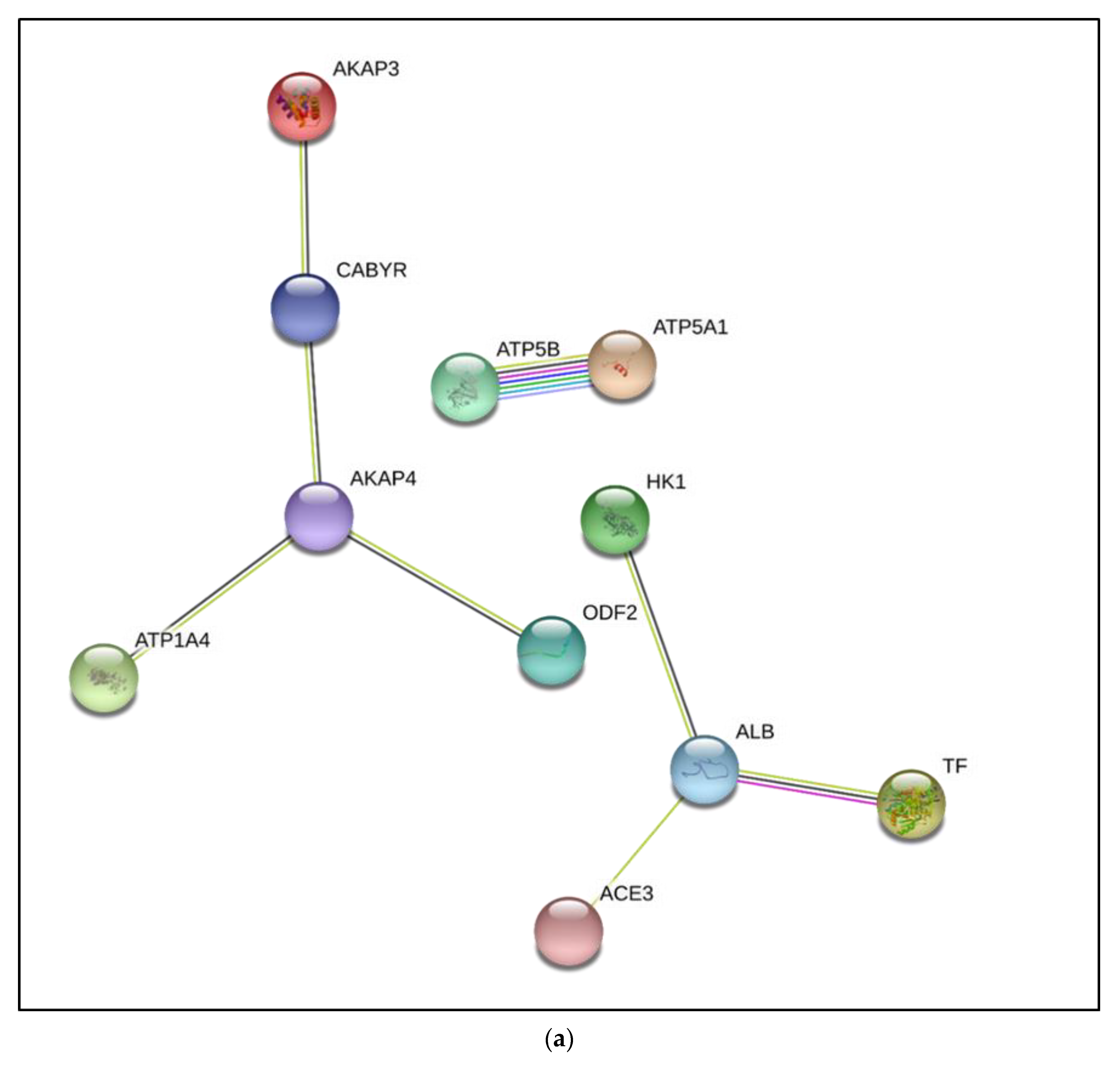
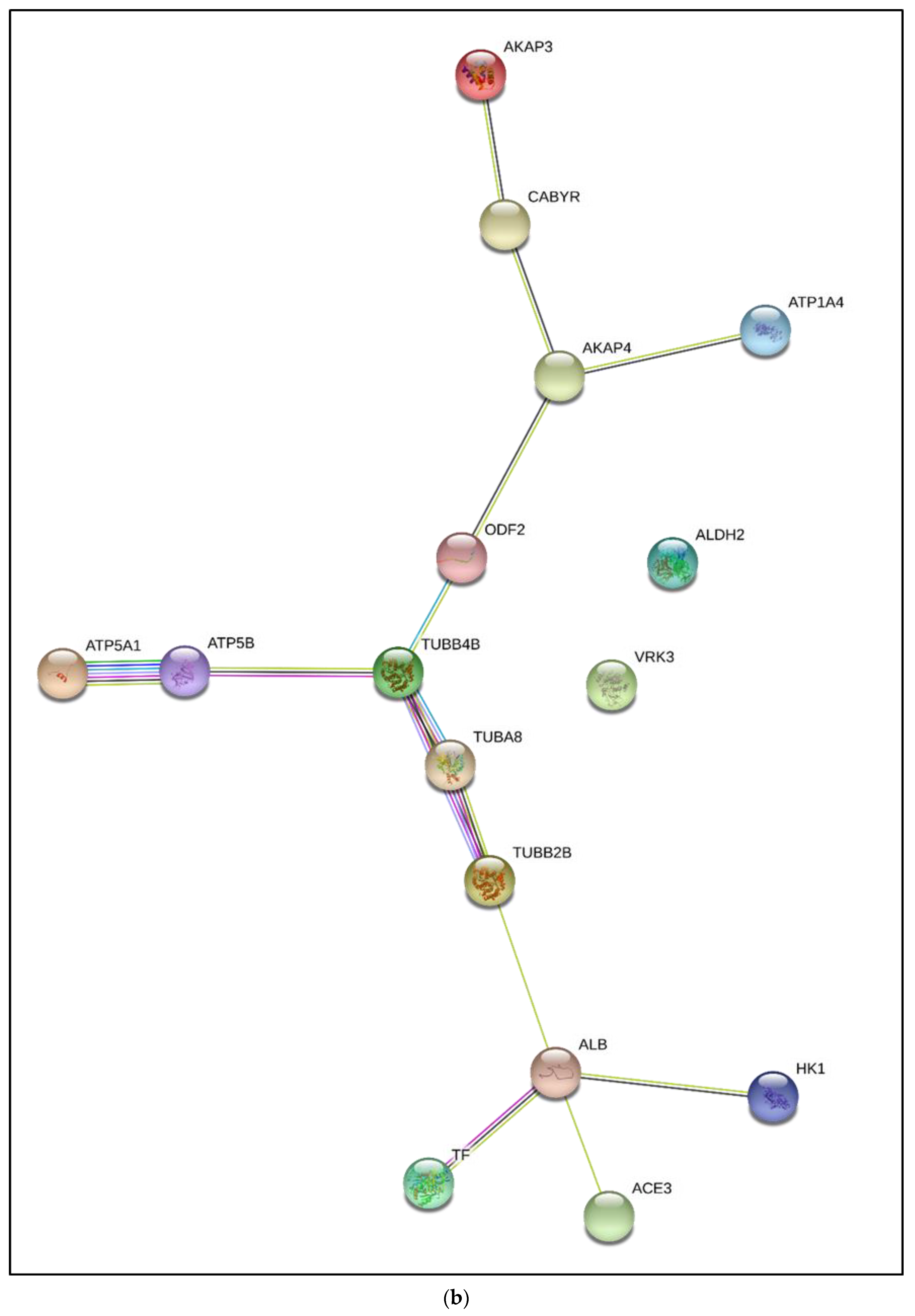
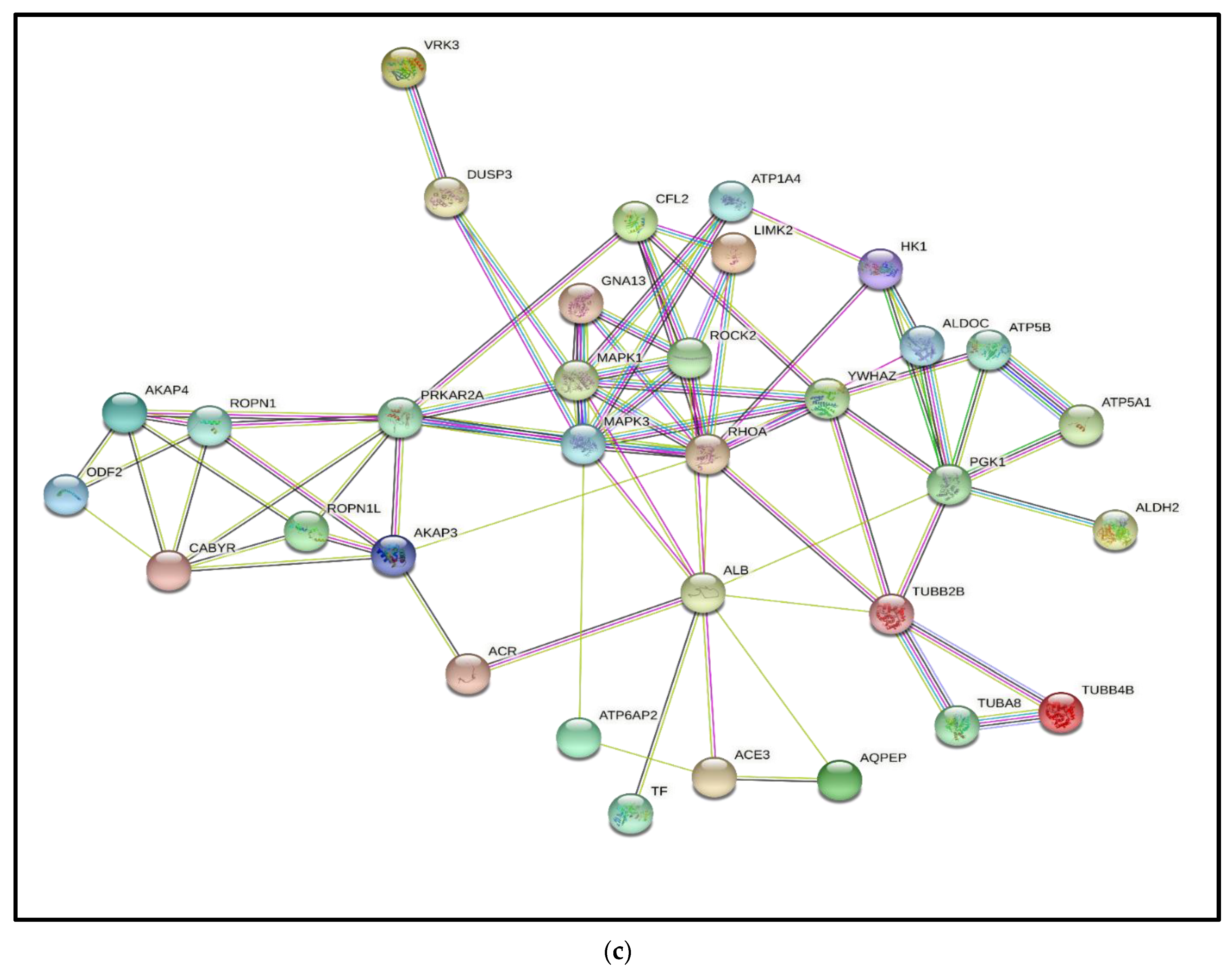
| Accession Number | Protein Name | Gene Name and GO Terms | Mean (±SD) Normalized Spectral Count in Fresh Sperm | Mean (±SD) Normalized Spectral Count in Capacitated Sperm | |
|---|---|---|---|---|---|
| F1MYH5 | A-Kinase anchoring protein 4 | AKAP4 | Protein binding; Protein localization; Scaffold/adaptor | 307.14 ± 39.39 a | 205.90 ± 38.45 b |
| Q2T9U2 | Outer dense fiber protein 2 | ODF2 | Developmental protein; Spermatogenesis | 141.85 ± 28.22 | 100 ± 26.32 |
| G3X6N3 | Serotransferrin | TF | Iron binding; Iron ion transport and localization; ERK1 and ERK2 cascade | 120.30 ± 36.40 | 87.80 ± 15.18 |
| F1MJS8 | A-Kinase anchoring protein 3 | AKAP3 | Protein binding; Protein localization; Scaffold/adaptor | 107.90 ± 19.37 | 73.38 ± 19.20 |
| A0A140T897 | Serum albumin | ALB | Chaperone binding; Zinc ion binding and localization | 250.17 ± 29.10 | 195.32 ± 15.72 |
| P00829 | Mitochondrial ATP synthase beta subunit | ATP5F1B | ATPase activity; Mitochondrial transmembrane transport; Cellular metabolic process; ATP synthesis | 26.26 ± 2.47 | 22.61 ± 5.90 |
| E1B8N5 | Testis-Specific isoform of Na+/K+-ATPase | ATP1A4 | ATPase activity; Ion homeostasis and localization | 25.85 ± 4.37 | 22.82 ± 6.5 |
| P27595 | Hexokinase | HK1 | Transferase activity; ATP binding; Glucose homeostasis; Pentose phosphate pathway | 80.57 ± 17.16 | 55.14 ± 10.31 |
| Q32L61 | Calcium binding tyrosine phosphorylation regulated | CABYR | Calcium ion binding; Sperm capacitation | 30.10 ± 4.22 | 28.16 ± 3.90 |
| P19483 | Mitochondrial ATP synthase subunit α | ATP5A1 | ATP synthase activity; Cellular metabolic process | 15.12 ± 4.40 | 10.69 ± 3.13 |
| Accession Number | Protein Name | Gene Name GO Terms | Mean (±SD) Normalized Spectral Count in Fresh Sperm | Mean (±SD) Normalized Spectral Count in Capacitated Sperm | |
|---|---|---|---|---|---|
| P20000 | Aldehyde dehydrogenase, mitochondrial | ALDH2 | Oxidoreductase; NAD binding; 5-Hydroxytryptamine degradation | 0 ± 0 | 15.12 ± 3.66 |
| Q2YDN8 | Inactive serine/threonine-protein kinase | VRK3 | Protein phosphorylation; Vesicle-mediated transport signal transduction; Membrane organization | 0 ± 0 | 10.10 ± 3.45 |
| Q3MHM5 | Tubulin-beta 4B chain | TUBB4B | Cytoskeleton; Microtubule cytoskeletal organization; Cytoskeletal regulation by Rho GTPase | 0 ± 0 | 150.90 ± 20.13 |
| Q2HJB8 | Tubulin-alpha 8 chain | TUBA8 | Cytoskeleton; Microtubule cytoskeletal organization | 0 ± 0 | 80.92 ± 19.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojaghi, M.; Varghese, J.; Kastelic, J.P.; Thundathil, J.C. Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation. Curr. Issues Mol. Biol. 2022, 44, 449-469. https://doi.org/10.3390/cimb44010031
Ojaghi M, Varghese J, Kastelic JP, Thundathil JC. Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation. Current Issues in Molecular Biology. 2022; 44(1):449-469. https://doi.org/10.3390/cimb44010031
Chicago/Turabian StyleOjaghi, Mina, Jacob Varghese, John P. Kastelic, and Jacob C. Thundathil. 2022. "Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation" Current Issues in Molecular Biology 44, no. 1: 449-469. https://doi.org/10.3390/cimb44010031
APA StyleOjaghi, M., Varghese, J., Kastelic, J. P., & Thundathil, J. C. (2022). Characterization of the Testis-Specific Angiotensin Converting Enzyme (tACE)-Interactome during Bovine Sperm Capacitation. Current Issues in Molecular Biology, 44(1), 449-469. https://doi.org/10.3390/cimb44010031





