The Role of OXT, OXTR, AVP, and AVPR1a Gene Expression in the Course of Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Safety and Exclusion Criteria
2.3. Method for Assessing Schizophrenia Severity
2.4. Sample Collection
2.5. Identification of Serum Protein Concentration
2.5.1. Determination of Protein Concentration
2.5.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Evaluation of Expression of Selected Genes at mRNA Level
2.6.1. Total RNA Isolation
2.6.2. Isolated RNA Quality Analysis
2.6.3. RT-PCR Reverse Transcription
2.6.4. Real-Time PCR Reaction
2.6.5. Statistical Analysis
3. Results
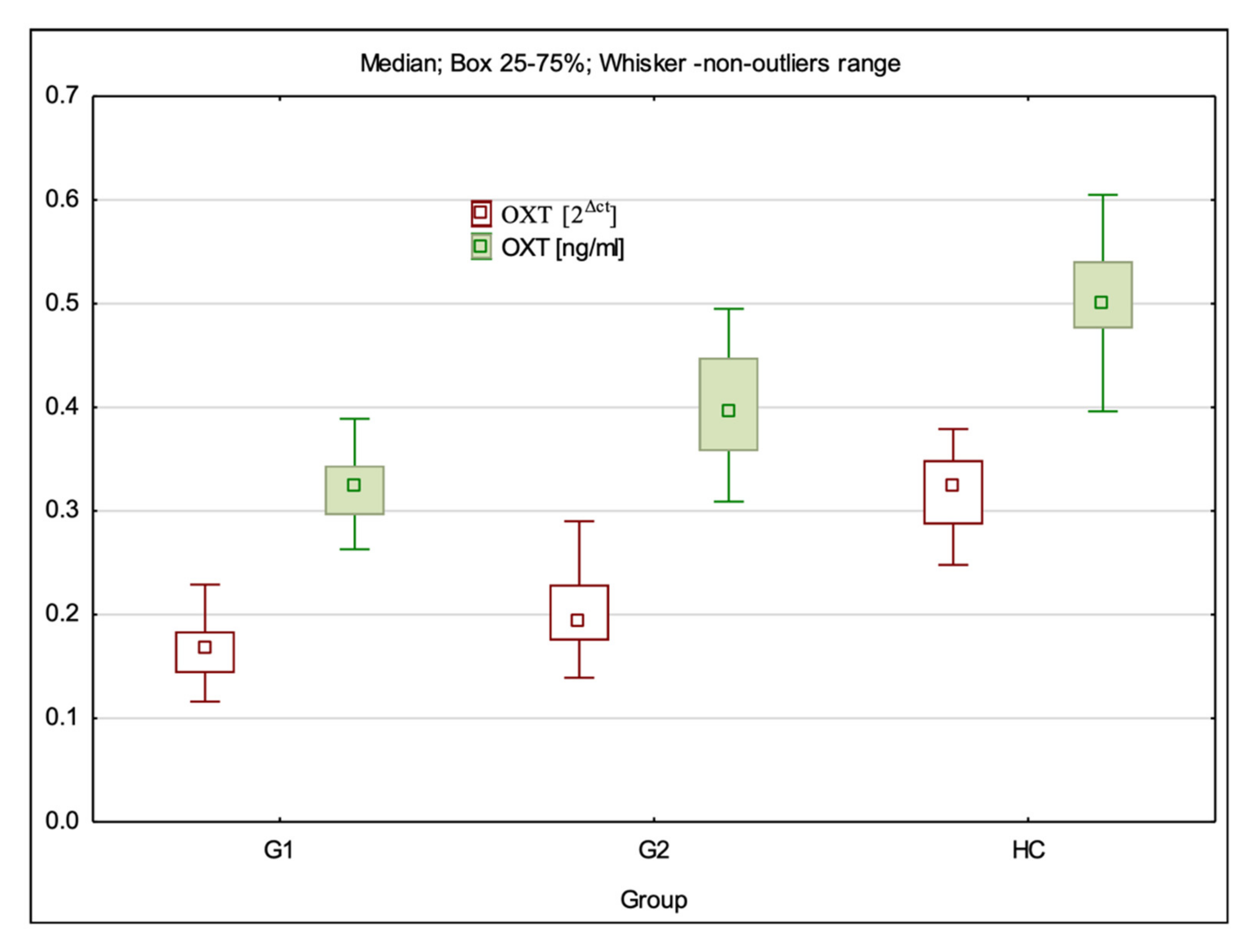
3.1. OXT Gene Expression at mRNA and Protein Levels
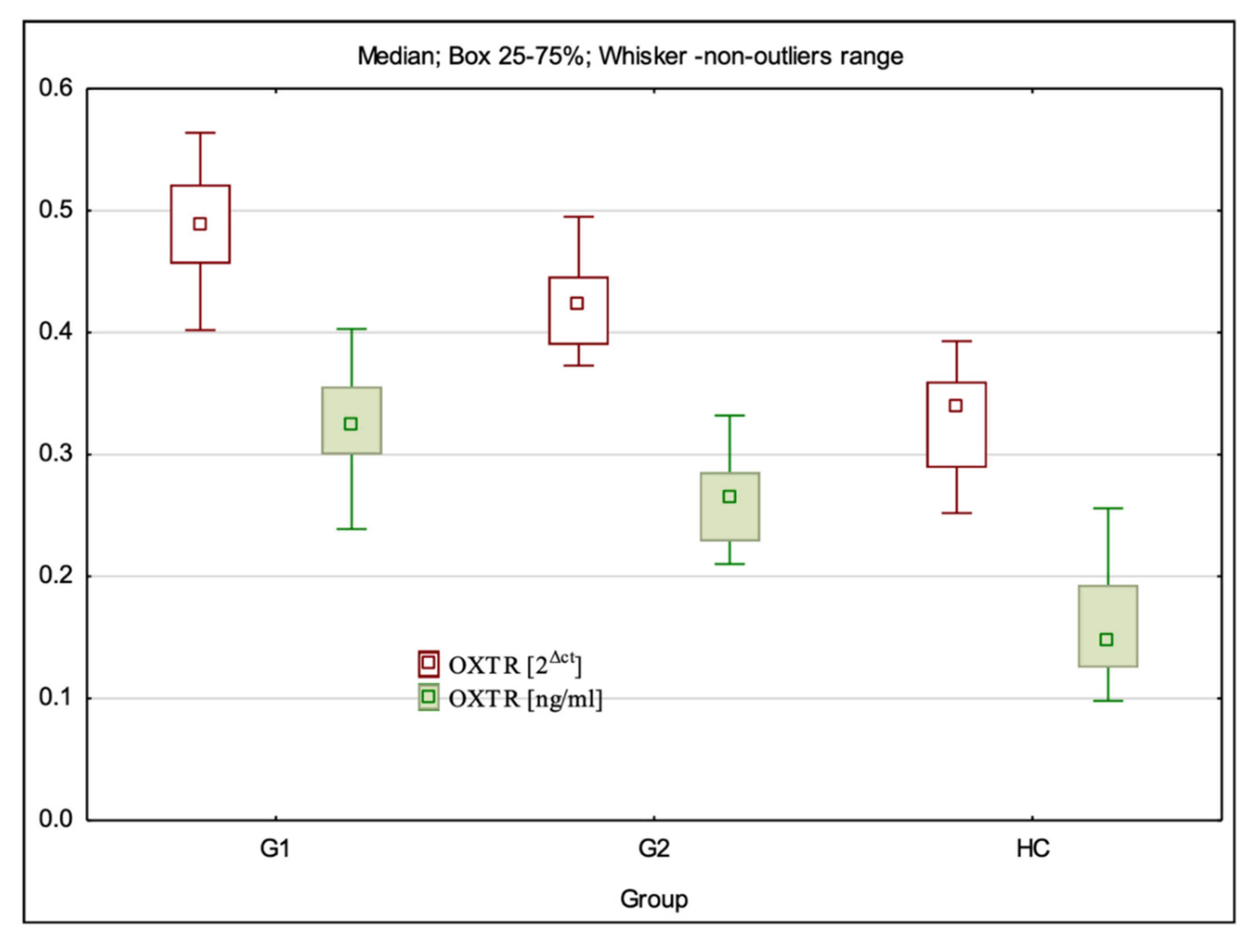
3.2. OXTR Gene Expression at mRNA and Protein Levels
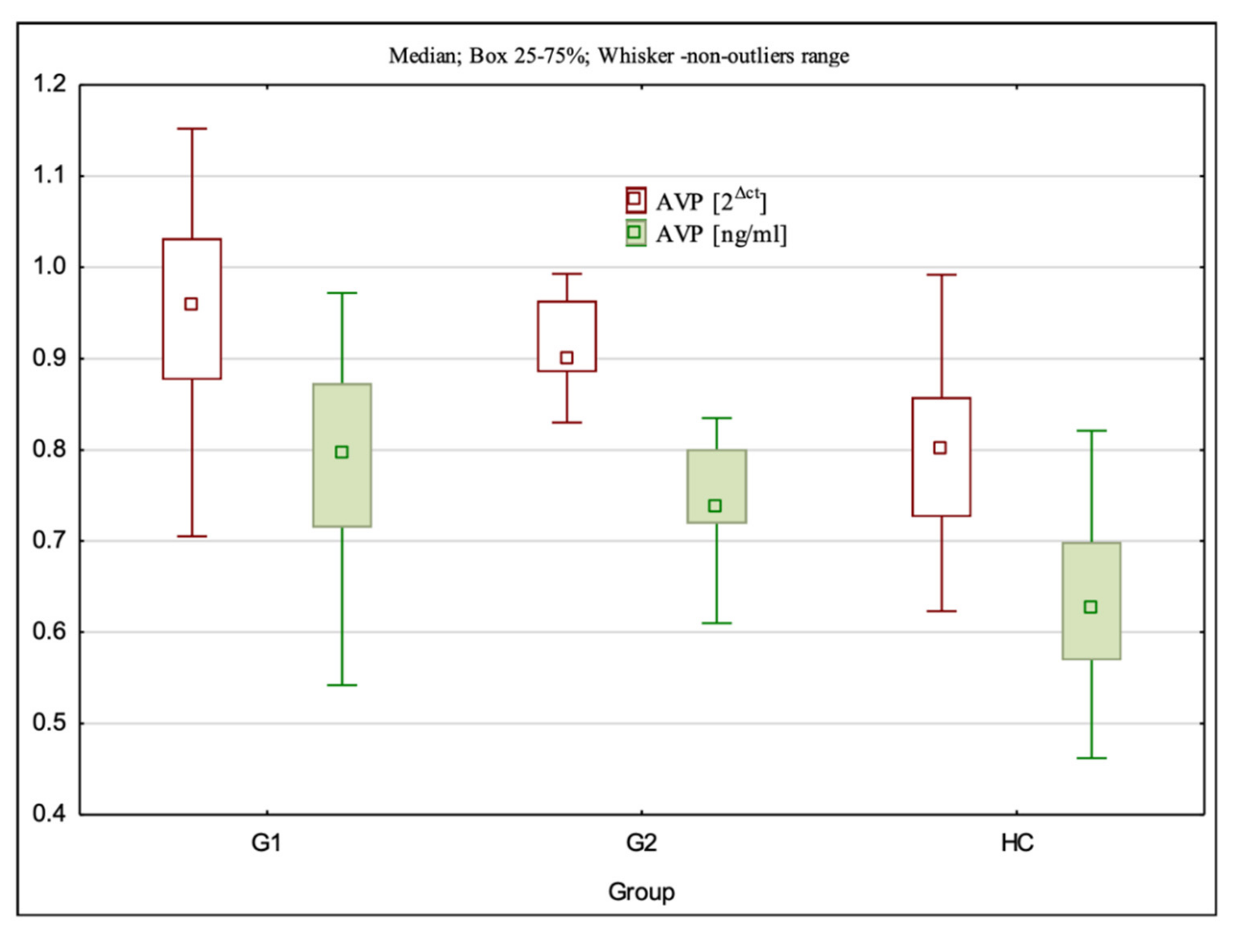
3.3. AVP Gene Expression at the mRNA and Protein Levels
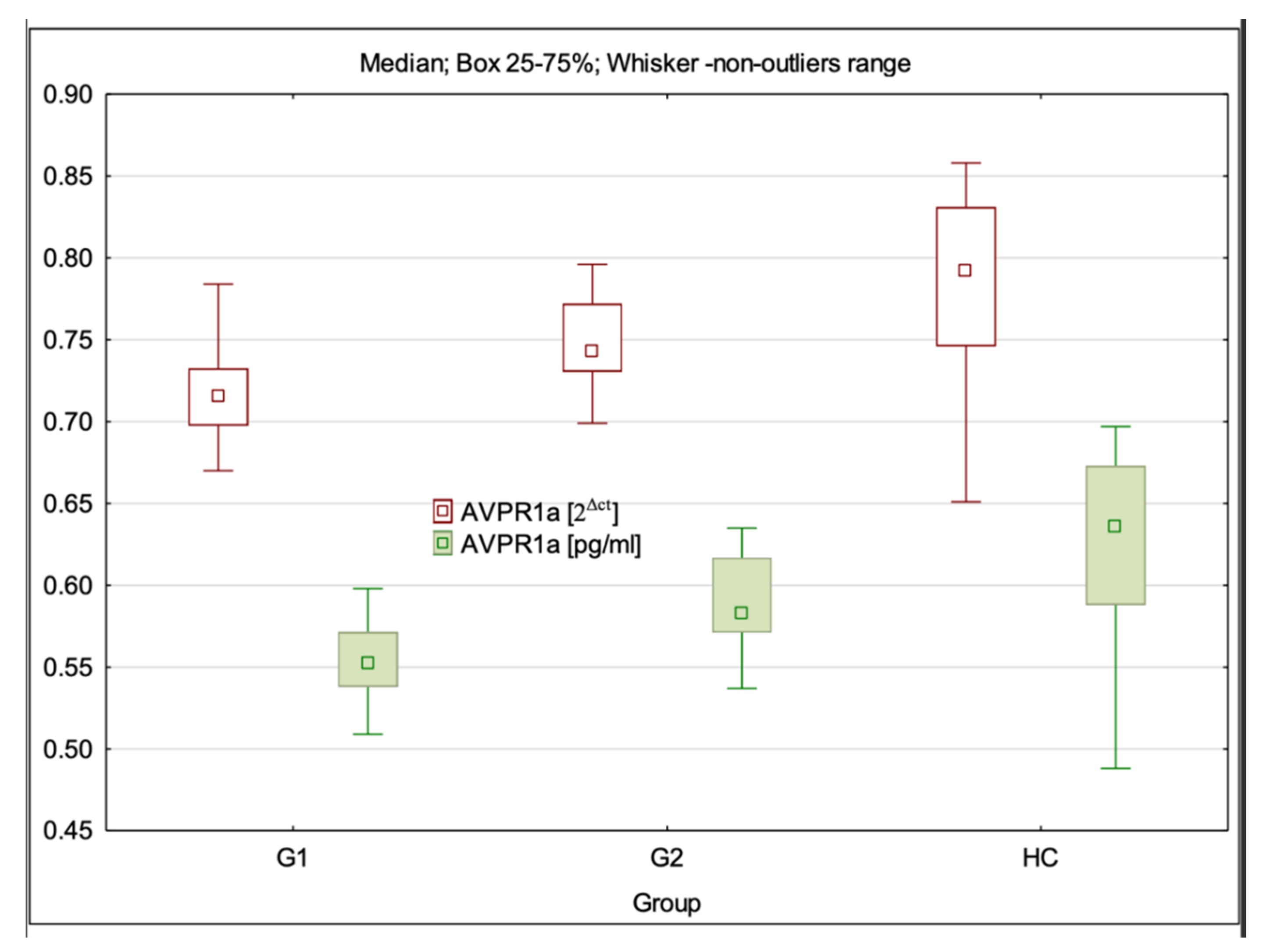
3.4. AVPR1a Gene Expression at mRNA and Protein Levels
3.5. Analysis of Correlation of OXT, OXTR, AVP, and AVPR1a Gene Expression at mRNA and Protein Levels with the Severity of Schizophrenia Symptoms Assessed by PANSS and CDSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings from the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A Systematic Review and Meta-Analysis of Recovery in Schizophrenia. Schizophr. Bull. 2012, 39, 1296–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.; Farh, K.H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H.; et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421. [Google Scholar] [CrossRef] [Green Version]
- Van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Mirnics, K. Neurodevelopment, GABA System Dysfunction, and Schizophrenia. Neuropsychopharmacology 2014, 40, 190–206. [Google Scholar] [CrossRef] [Green Version]
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Lewis, D.A.; Levitt, P. Schizophrenia as a Disorder of Neurodevelopment. Annu. Rev. Neurosci. 2002, 25, 409–432. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Weinberger, D.R. Future of Days Past: Neurodevelopment and Schizophrenia. Schizophr. Bull. 2017, 43, 1164–1168. [Google Scholar] [CrossRef]
- Buoli, M.; Serati, M.; Caldiroli, A.; Cremaschi, L.; Altamura, A.C. Neurodevelopmental Versus Neurodegenerative Model of Schizophrenia and Bipolar Disorder: Comparison with Physiological Brain Development and Aging. Psychiatr. Danub. 2017, 29, 24–27. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef]
- Glahn, D.C.; Kent, J.W.; Sprooten, E.; Diego, V.P.; Winkler, A.M.; Curran, J.E.; McKay, D.R.; Knowles, E.E.; Carless, M.A.; Goring, H.H.H.; et al. Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging. Proc. Natl. Acad. Sci. USA 2013, 110, 19006–19011. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.; Chen, C.-H.; Lane, H.-Y. Oxytocin in Schizophrenia: Pathophysiology and Implications for Future Treatment. Int. J. Mol. Sci. 2021, 22, 2146. [Google Scholar] [CrossRef]
- Bradley, E.R.; Woolley, J.D. Oxytocin effects in schizophrenia: Reconciling mixed findings and moving forward. Neurosci. Biobehav. Rev. 2017, 80, 36–56. [Google Scholar] [CrossRef]
- Parker, K.J.; Buckmaster, C.; Schatzberg, A.F.; Lyons, D.M. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 2005, 30, 924–929. [Google Scholar] [CrossRef]
- Storm, E.E.; Tecott, L.H. Social Circuits: Peptidergic Regulation of Mammalian Social Behavior. Neuron 2005, 47, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Legros, J.-J. Inhibitory effect of oxytocin on corticotrope function in humans: Are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology 2001, 26, 649–655. [Google Scholar] [CrossRef]
- Zak, P.J.; Stanton, A.A.; Ahmadi, S. Oxytocin Increases Generosity in Humans. PLoS ONE 2007, 2, e1128. [Google Scholar] [CrossRef]
- Oettl, L.-L.; Ravi, N.; Schneider, M.; Scheller, M.F.; Schneider, P.; Mitre, M.; Gouveia, M.D.S.; Froemke, R.; Chao, M.; Young, W.S.; et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Gläscher, J.; Büchel, C.; Braus, D.F.; Herpertz, S.C. Oxytocin Attenuates Amygdala Responses to Emotional Faces Regardless of Valence. Biol. Psychiatry 2007, 62, 1187–1190. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Michel, A.; Berger, C.; Herpertz, S.C. Oxytocin Improves “Mind-Reading” in Humans. Biol. Psychiatry 2007, 61, 731–733. [Google Scholar] [CrossRef]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Dumais, K.M.; Veenema, A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocr. 2015, 40, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.R.; George, K.; Walton, J.C.; Orr, S.P.; Benson, J. Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. USA 2006, 103, 7889–7894. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Woolley, J.D.; Chuang, B.; Fussell, C.; Scherer, S.; Biagianti, B.; Fulford, D.; Mathalon, D.H.; Vinogradov, S. Intranasal oxytocin increases facial expressivity, but not ratings of trustworthiness, in patients with schizophrenia and healthy controls. Psychol. Med. 2017, 47, 1311–1322. [Google Scholar] [CrossRef]
- Cherian, K.; Schatzberg, A.F.; Keller, J. HPA axis in psychotic major depression and schizophrenia spectrum disorders: Cortisol, clinical symptomatology, and cognition. Schizophr. Res. 2019, 213, 72–79. [Google Scholar] [CrossRef]
- Jobst, A.; Dehning, S.; Ruf, S.; Notz, T.; Buchheim, A.; Henning-Fast, K.; Meißner, D.; Meyer, S.; Bondy, B.; Müller, N.; et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2013, 26, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Aydın, O.; Lysaker, P.H.; Balıkçı, K.; Ünal-Aydın, P.; Esen-Danacı, A. Associations of oxytocin and vasopressin plasma levels with neurocognitive, social cognitive and meta cognitive function in schizophrenia. Psychiatry Res. 2018, 270, 1010–1016. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Li, C.; Tao, H.; Yang, X.; Zhang, X.; Wang, X. Altered Expression of Glucocorticoid Receptor and Neuron-Specific Enolase mRNA in Peripheral Blood in First-Episode Schizophrenia and Chronic Schizophrenia. Front. Psychiatry 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G. HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Szafranski, T. Calgary Depression Scale for Schizophrenia. In Advances in Psychiatry and Neurology; Institute of Psychiatry and Neurology: Warsaw, Poland, 1997; pp. 333–343. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Valstad, M.; Alvares, G.A.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The relationship between central and peripheral oxytocin concentrations: A systematic review and meta-analysis protocol. Syst. Rev. 2016, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Walss-Bass, C.; Fernandes, J.M.; Roberts, D.L.; Service, H.; Velligan, D. Differential correlations between plasma oxytocin and social cognitive capacity and bias in schizophrenia. Schizophr. Res. 2013, 147, 387–392. [Google Scholar] [CrossRef]
- Sasayama, D.; Hattori, K.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; Kunugi, H. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophr. Res. 2012, 139, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, A.J.; Lieberman, J.A.; Jarskog, L.F. Oxytocin, Dopamine, and the Amygdala: A Neurofunctional Model of Social Cognitive Deficits in Schizophrenia. Schizophr. Bull. 2011, 37, 1077–1087. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Sertic, S.; Reversi, A.; Chini, B. Intracellular trafficking of the human oxytocin receptor: Evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am. J. Physiol. Metab. 2009, 296, E532–E542. [Google Scholar] [CrossRef] [Green Version]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tao, H.; Yang, X.; Huang, K.; Zhang, X.; Li, C. Decreased Serum Oxytocin and Increased Homocysteine in First-Episode Schizophrenia Patients. Front. Psychiatry 2019, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Tang, Y.; Wei, Q.; Lang, B.; Tao, H.; Zhang, X.; Liu, Y.; Tang, A. Up-regulated expression of oxytocin mRNA in peripheral blood lymphocytes from first-episode schizophrenia patients. Oncotarget 2017, 8, 78882–78889. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.; Marlow-O’Connor, M.; Torres, I.; Carter, C. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr. Res. 2008, 98, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Guzel, D.; Yazici, A.B.; Pek, T.M.; Doganay, S.; Simsek, A.B.S.; Sağlam, K.; Turan, C.; Yazici, E. Atrial natriuretic peptide and posterior pituitary neurohormone changes in patients with acute schizophrenia. Neuropsychiatr. Dis. Treat. 2018, 14, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Balikci, K.; Aydin, O.; Tas, C.; Danaci, A.E. Oxytocin and social cognition in patients with schizophrenia: Comparison with healthy siblings and healthy controls. Psychiatry Clin. Psychopharmacol. 2017, 28, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.H.; Wehring, H.J.; Demyanovich, H.; Carter, C.S.; Pournajafi-Nazarloo, H.; Feldman, S.M.; Earl, A.K.; August, S.; Gold, J.M.; Kelly, D.L. Peripheral oxytocin and vasopressin are associated with clinical symptom severity and cognitive functioning in midlife women with chronic schizophrenia. Schizophr. Res. 2018, 195, 409–411. [Google Scholar] [CrossRef]
- Aydın, O.; Balıkçı, K.; Taş, C.; Ünal-Aydın, P.; Taneli, F.; Esen-Danacı, A. Assessing the relationship between attachment, parental attitude and plasma oxytocin in schizophrenia patients and their unaffected siblings. Nord. J. Psychiatry 2019, 73, 51–57. [Google Scholar] [CrossRef]
- Feifel, D.; Macdonald, K.; Nguyen, A.; Cobb, P.; Warlan, H.; Galangue, B.; Minassian, A.; Becker, O.; Cooper, J.; Perry, W.; et al. Adjunctive Intranasal Oxytocin Reduces Symptoms in Schizophrenia Patients. Biol. Psychiatry 2010, 68, 678–680. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Gibson, C.M.; Rau, S.W.; Salimi, K.; Smedley, K.L.; Casey, R.L.; Leserman, J.; Jarskog, L.F.; Penn, D.L. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res. 2011, 132, 50–53. [Google Scholar] [CrossRef]
- Cardaillac, C.; Rua, C.; Simon, E.G.; El-Hage, W. Oxytocin and postpartum depression. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 786–795. [Google Scholar] [CrossRef]
- Thul, T.A.; Corwin, E.J.; Carlson, N.S.; Brennan, P.A.; Young, L.J. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology 2020, 120, 104793. [Google Scholar] [CrossRef]
- McQuaid, R.J.; McInnis, O.A.; Abizaid, A.; Anisman, H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014, 45, 305–322. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Hansenne, M.; Fuchs, S.; Pitchot, W.; Maréchal, P.; Pequeux, C.; Ansseau, M.; Legros, J. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007, 32, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Ozsoy, S.; Esel, E.; Kula, M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009, 169, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Kenna, H.A.; Zeitzer, J.M.; Keller, J.; Blasey, C.M.; Amico, J.A.; Schatzberg, A.F. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010, 178, 359–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.M.; Wei, Q.; Jiang, Z.H.; Shi, Y.Y.; Zhang, Y.H.; Shi, H.J. The role of serum oxytocin levels in the second trimester in regulating prenatal anxiety and depression: A sample from Shanghai Maternal-Child Pairs Cohort study. J. Affect. Disord. 2020, 264, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K.; Alster, P.; Svensson, T.H. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology 1992, 109, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.F.; Hu, S.-B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef]
- Albers, H.E. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocr. 2015, 36, 49–71. [Google Scholar] [CrossRef] [Green Version]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, R.; Gagliardi, L.; Vicennati, V.; Gambineri, A.; Colitta, D.; Ceroni, L.; Casimirri, F. ACTH and cortisol response to combined corticotropin releasing hormone-arginine vasopressin stimulation in obese males and its relationship to body weight, fat distribution and parameters of the metabolic syndrome. Int. J. Obes. 1999, 23, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-D.; Burton, K.J.; Zhang, C.; Hu, S.-B.; Zhou, Q.-Y. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R824–R830. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Harris, M.S.; Hill, S.K.; Reilly, J.L.; Sweeney, J.A. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr. Res. 2013, 146, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Geng, C.-H.; Wang, C.; Yang, J.; Wang, H.; Ma, R.-Q.; Liu, X.; Wang, C.-H. Arginine vasopressin improves the memory deficits in Han Chinese patients with first-episode schizophrenia. Peptides 2017, 97, 8–15. [Google Scholar] [CrossRef]
- Hosseini, S.M.R.; Farokhnia, M.; Rezaei, F.; Gougol, A.; Yekehtaz, H.; Iranpour, N.; Salehi, B.; Tabrizi, M.; Tajdini, M.; Ghaleiha, A.; et al. Intranasal desmopressin as an adjunct to risperidone for negative symptoms of schizophrenia: A randomized, double-blind, placebo-controlled, clinical trial. Eur. Neuropsychopharmacol. 2014, 24, 846–855. [Google Scholar] [CrossRef]
- Brambilla, F.; Bondiolotti, G.; Maggioni, M.; Sciascia, A.; Grillo, W.; Sanna, F.; Latina, A.; Picotti, G. Vasopressin (DDAVP) Therapy in Chronic Schizophrenia: Effects on Negative Symptoms and Memory. Neuropsychobiology 1988, 20, 113–119. [Google Scholar] [CrossRef]
- Wójciak, P.; Remlinger-Molenda, A.; Rybakowski, J. The role of oxytocin and vasopressin in central nervous system activity and mental disorders. Psychiatr. Polska 2013, 46, 1043–1052. [Google Scholar]
| G1 | G2 | HC | p-Value | |
|---|---|---|---|---|
| OXT 2Δct | 0.17 (0.12–0.23) ± 0.03 * | 0.19 (0.14–0.29) ± 0.03 * | 0.32 (0.25–0.38) ± 0.04 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2 p = 0.008 |
| OXTR 2Δct | 0.49(0.40–0.56) ± 0.04 * | 0.42 (0.37–0.50) ± 0.03 * | 0.34 (0.17–0.39) ± 0.05 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2—p = 0.000 |
| AVP 2Δct | 0.96 (0.71–1.15) ± 0.11 ** | 0.90 (0.83–0.99) ± 0.05 ** | 0.80 (0.62–0.99) ± 0.09 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2—p > 0.05 |
| AVPR1a 2Δct | 0.72 (0.67–0.79) ± 0.03 * | 0.74 (0.70–0.80) ± 0.03 *** | 0.79 (0.65–0.86) ± 0.06 | G1:HC—p < 0.001; G2:HC—p > 0.05; G1:G2—p = 0.001 |
| G1 | G2 | HC | p-Value | |
|---|---|---|---|---|
| OXT ng/mL | 0.32 (0.26–0.39) ± 0.03 * | 0.40 (0.31–0.50) ± 0.05 * | 0.50 (0.40–0.61) ± 0.05 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2 p < 0.001 |
| OXTR ng/mL | 0.32 (0.24–0.57) ± 0.07 * | 0.26 (0.21–0.33) ± 0.03 * | 0.15 (0.10–0.26) ± 0.05 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2 p < 0.001 |
| AVP ng/mL | 0.89 (0.54–0.97) ± 0.11 ** | 0.74 (0.61–0.84) ± 0.05 ** | 0.63 (0.46–0.82) ± 0.09 | G1:HC—p < 0.001; G2:HC—p < 0.001; G1:G2 p > 0.05 |
| AVPR1a pg/mL | 0.55 (0.51–0.75) ± 0.04 * | 0.58 (0.54–0.64) ± 0.03 *** | 0.64 (0.49–0.70) ± 0.03 | G1:HC—p < 0.001; G2:HC—p > 0.05; G1:G2 p = 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broniarczyk-Czarniak, M.; Szemraj, J.; Śmigielski, J.; Gałecki, P. The Role of OXT, OXTR, AVP, and AVPR1a Gene Expression in the Course of Schizophrenia. Curr. Issues Mol. Biol. 2022, 44, 336-349. https://doi.org/10.3390/cimb44010025
Broniarczyk-Czarniak M, Szemraj J, Śmigielski J, Gałecki P. The Role of OXT, OXTR, AVP, and AVPR1a Gene Expression in the Course of Schizophrenia. Current Issues in Molecular Biology. 2022; 44(1):336-349. https://doi.org/10.3390/cimb44010025
Chicago/Turabian StyleBroniarczyk-Czarniak, Marta, Janusz Szemraj, Janusz Śmigielski, and Piotr Gałecki. 2022. "The Role of OXT, OXTR, AVP, and AVPR1a Gene Expression in the Course of Schizophrenia" Current Issues in Molecular Biology 44, no. 1: 336-349. https://doi.org/10.3390/cimb44010025
APA StyleBroniarczyk-Czarniak, M., Szemraj, J., Śmigielski, J., & Gałecki, P. (2022). The Role of OXT, OXTR, AVP, and AVPR1a Gene Expression in the Course of Schizophrenia. Current Issues in Molecular Biology, 44(1), 336-349. https://doi.org/10.3390/cimb44010025








