Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. pGC Isolation and Culture
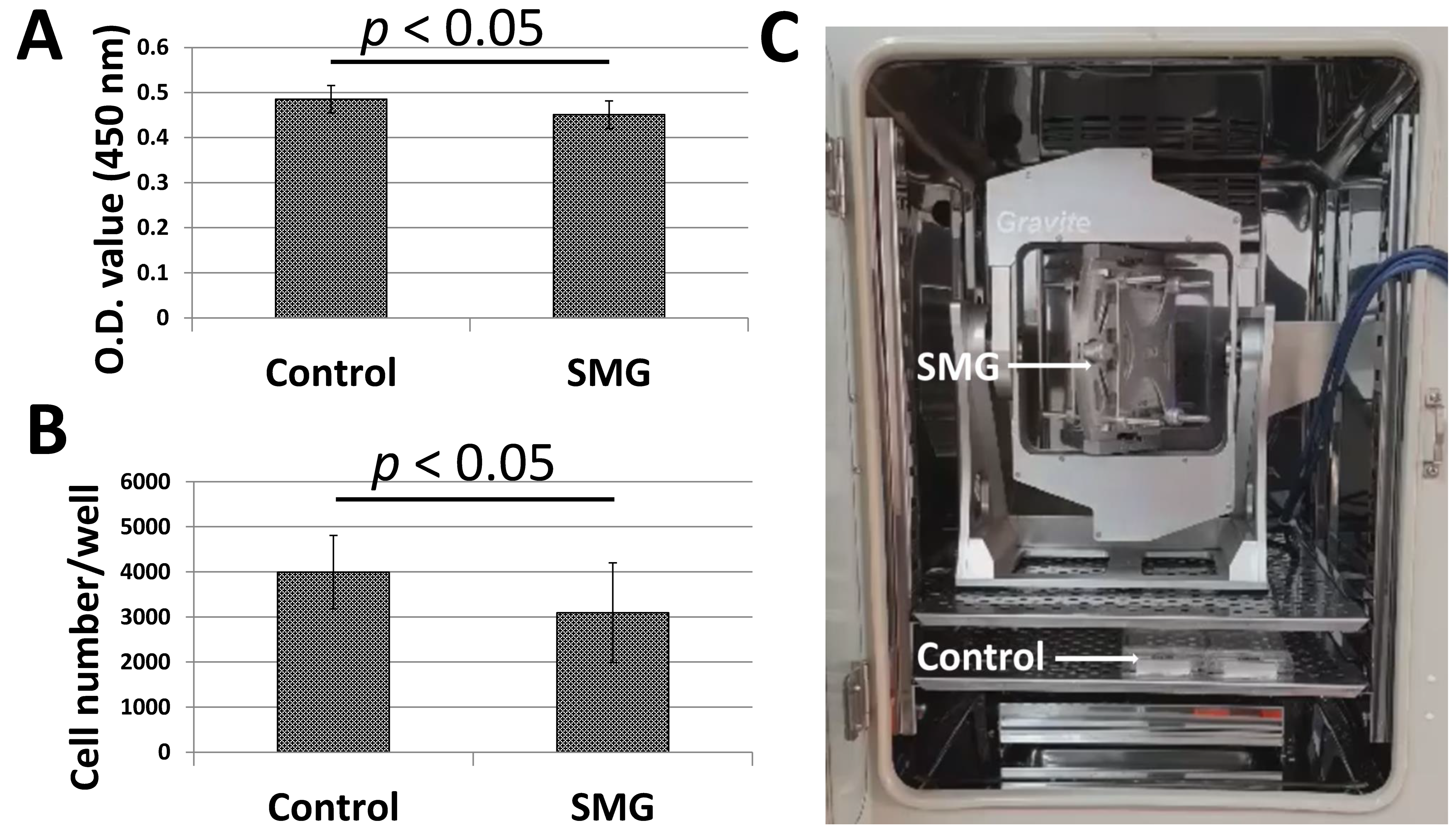
2.2. Microgravity Simulation
2.3. WST-1 Assay
2.4. Cell Density Determination
2.5. Flow Cytometry
2.6. Western Blot
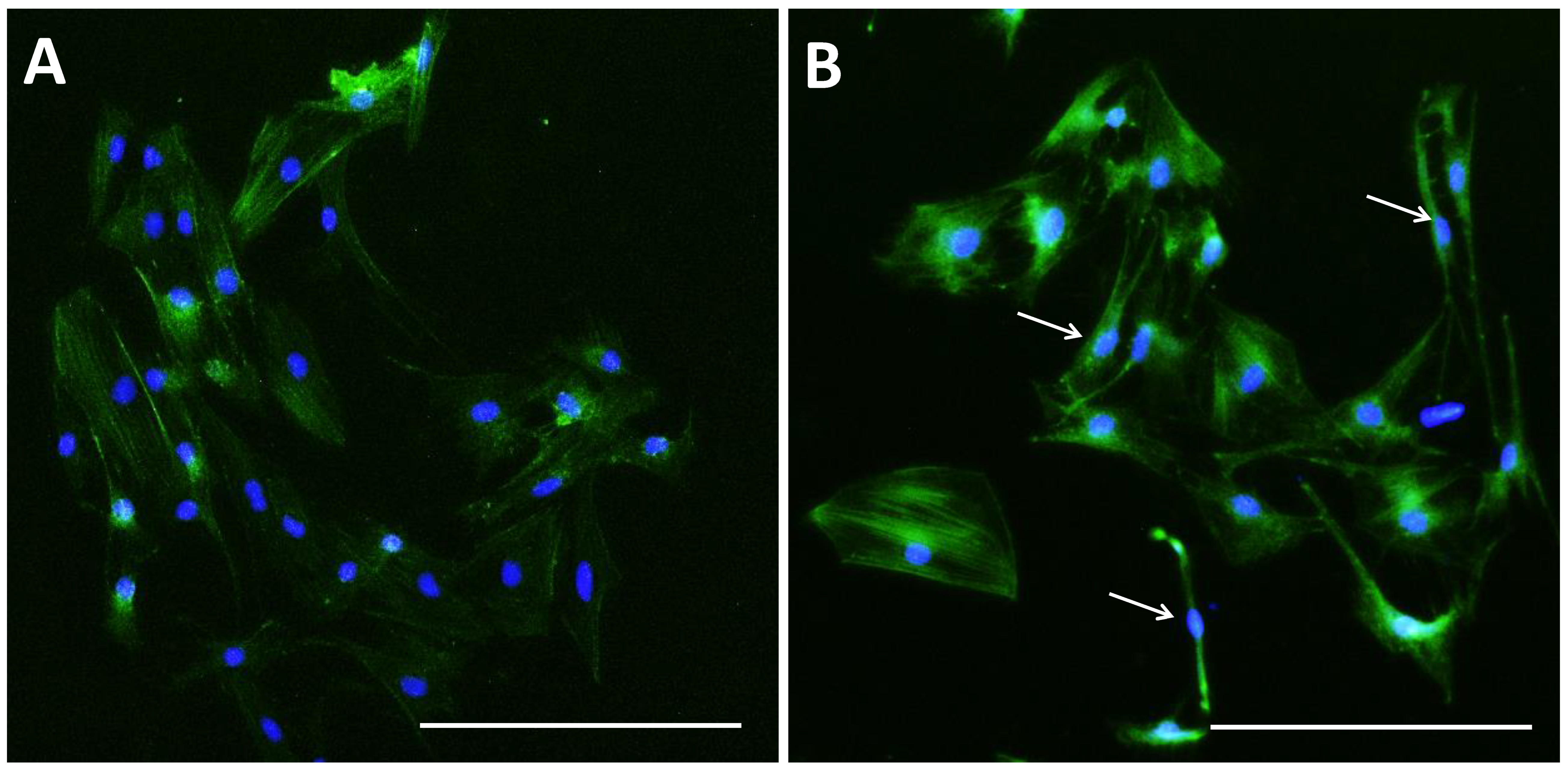
2.7. Microfilament Staining
2.8. Statistical Analysis
3. Results
3.1. SMG Inhibited the Proliferation of pGCs
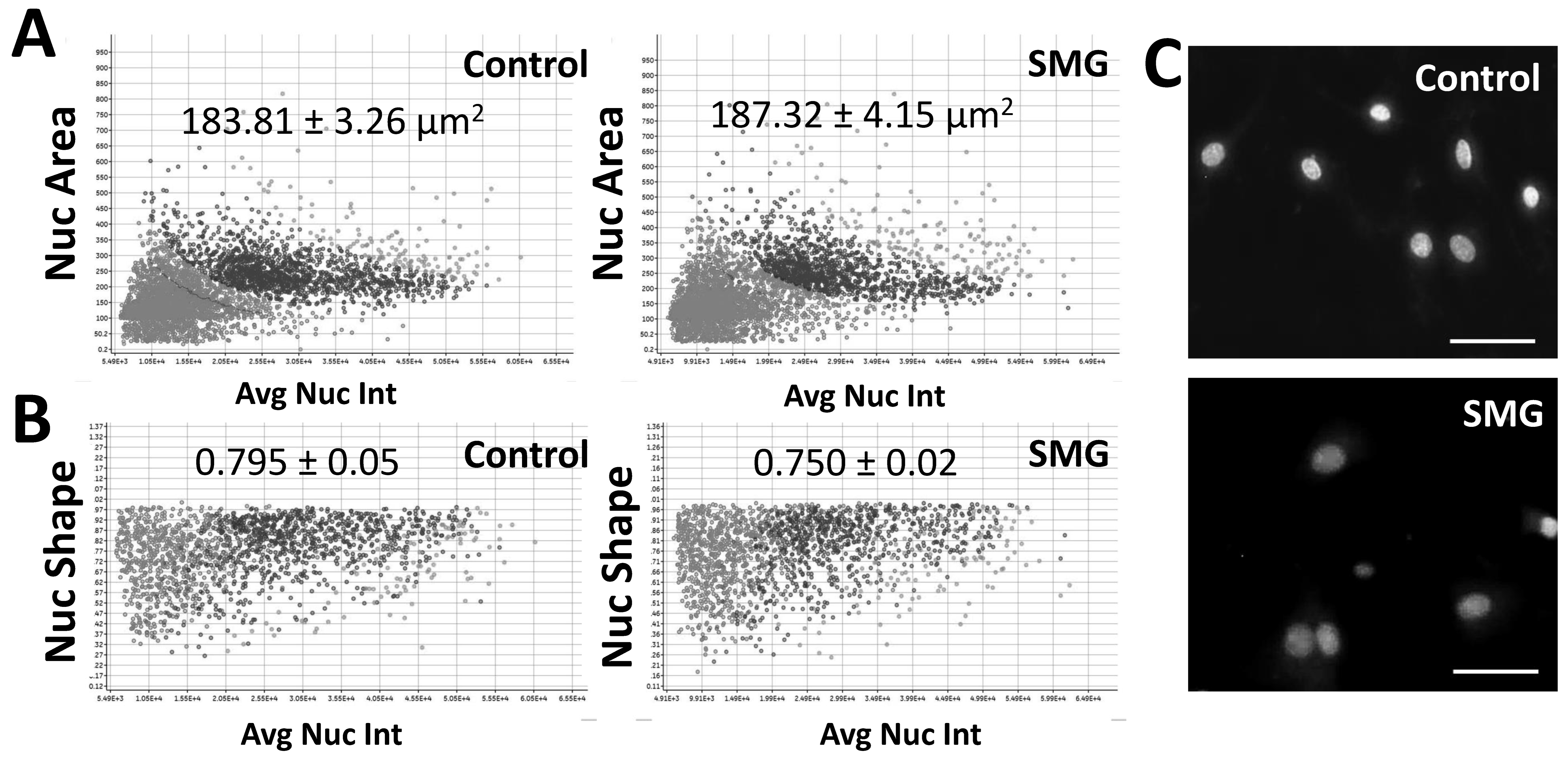
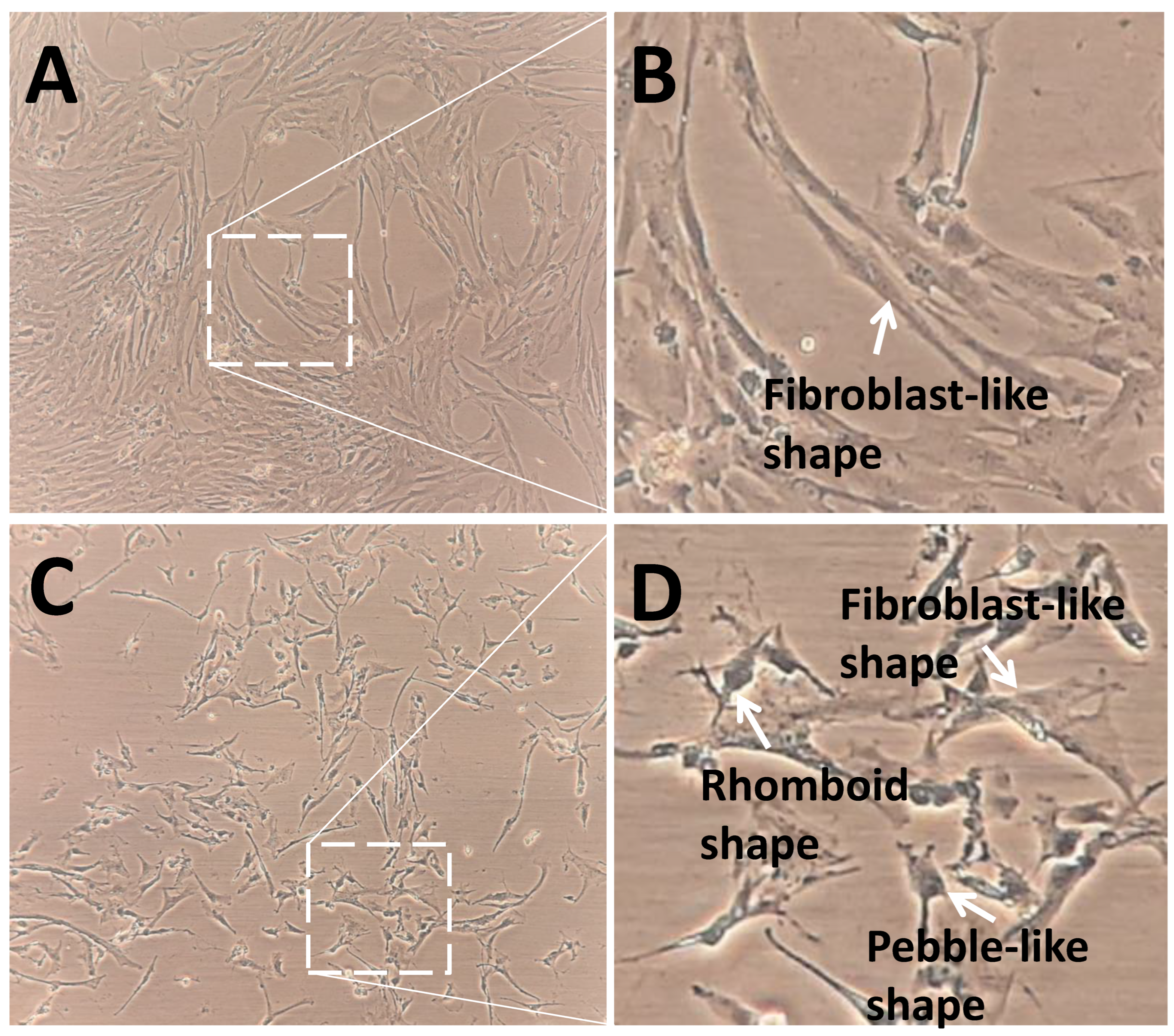
3.2. SMG Induced Morphological Changes in pGCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bizzarri, M.; Monici, M.; Van Loon, J.J.W.A. How Microgravity Affects the Biology of Living Systems. BioMed Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Sapp, W.J.; Philpott, D.E.; Williams, C.S.; Kato, K.; Stevenson, J.; Vasquez, M.; Serova, L.V. Effects of spaceflight on the spermatogonial population of rat seminiferous epithelium. FASEB J. 1990, 4, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.P.; Deaver, D.R.; Zirkin, B.R.; Grills, G.S.; Sapp, W.J.; Veeramachaneni, D.N.; Clemens, J.W.; Banerjee, S.D.; Folmer, J.; Gruppi, C.M.; et al. Effects of microgravity or simulated launch on testicular function in rats. J. Appl. Physiol. 1992, 73 (Suppl. 2), S174–S185. [Google Scholar] [CrossRef] [Green Version]
- Burden, H.W.; Zary, J.; Lawrence, I.E.; Jonnalagadda, P.; Davis, M.; Hodson, C.A. Effects of space flight on ovarian-hypophyseal function in postpartum rats. J Reprod Fertil. 1997, 109, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Tou, J.C.L.; Grindeland, R.E.; Wade, C.E. Effects of diet and exposure to hindlimb suspension on estrous cycling in Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E425–E433. [Google Scholar] [CrossRef] [Green Version]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of sex and gender on adaptations to space: Reproductive health. J. Womens Health 2014, 23, 967–7474. [Google Scholar] [CrossRef] [Green Version]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Grimm, D.; Strauch, S.; Erzinger, G.; Corydon, T.; Lebert, M.; Magnusson, N.; Infanger, M.; Richter, P.; Krüger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- Richards, J.S. Hormonal control of gene expression in the ovary. Endocr. Rev. 1994, 15, 725–751. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.; Petitte, J.; Stelmach, B.; et al. Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, S.C.; Stocco, C. Granulosa Cells. In Encyclopedia of Reproduction; Elsevier: Chicago, IL, USA, 2018; pp. 8–13. [Google Scholar]
- Panepinto, L.M. Miniature swine breeds used worldwide in research. In Advances in Swine in Biomedical Research; Tumbleson, M.E., Schook, L.B., Eds.; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- Kuzmuk, K.N.; Schook, L.B. Pig as a model for biomedical sciences. In The Genetics of the Pig; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Cambridge, MA, USA, 2011. [Google Scholar]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikkam, M.; Li, Y.; Mitchell, B.M.; Mason, D.E.; Freeman, L.C. Potassium Channel Antagonists Influence Porcine Granulosa Cell Proliferation, Differentiation, and Apoptosis1. Biol. Reprod. 2002, 67, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Barano, J.L.S.; Hammond, J.M. Serum-Free Medium Enhances Growth and Differentiation of Cultured Pig Granulosa Cells. Endocrinology 1985, 116, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Sun, A.; Li, H.; Tsinkgou, A.; Yu, J.; Ying, S.; Chen, Z.; Shi, Z. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody. Reprod. Biol. Endocrinol. 2015, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- Ożegowska, K.; Brązert, M.; Ciesiółka, S.; Nawrocki, M.J.; Kranc, W.; Celichowski, P.; Jankowski, M.; Bryja, A.; Jeseta, M.; Antosik, P.; et al. Genes Involved in the Processes of Cell Proliferation, Migration, Adhesion, and Tissue Development as New Potential Markers of Porcine Granulosa Cellular Processes In Vitro: A Microarray Approach. DNA Cell Biol. 2019, 38, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Opydo-Chanek, M.; Dupont, J.; Rak, A. In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3. Int. J. Mol. Sci. 2019, 20, 5816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Liu, C.; Zhan, X.; Li, J. Protegrin-1 Regulates Porcine Granulosa Cell Proliferation via the EGFR-ERK1/2/p38 Signaling Pathway in vitro. Front. Physiol. 2021, 12, 673777. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhu, C.; Feng, M.; Pan, B.; Zhang, S.; Zhan, X.; Chen, H.; Wang, B.; Li, J. Establishment of A Reversibly Inducible Porcine Granulosa Cell Line. Cells 2020, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tao, Q.; Shang, J.; Xu, Y.; Zhang, L.; Ma, Y.; Zhu, W.; Yang, M.; Ding, Y.; Yin, Z. MiR-26a promotes apoptosis of porcine granulosa cells by targeting the 3β-hydroxysteroid-Δ24-reductase gene. Asian-Australas. J. Anim. Sci. 2020, 33, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Y.; Wang, Y.; Li, Y.; He, Y.; Duan, J.; Xu, D.; Pei, Y.; Cheng, J.; Yang, L.; et al. Phospholipase C inhibits apoptosis of porcine primary granulosa cells cultured in vitro. J. Ovarian Res. 2019, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, L.; Liu, N.; Tang, X.; Guo, S.; Zhang, B.; Jiang, Z. Autophagy and Apoptosis of Porcine Ovarian Granulosa Cells During Follicular Development. Animals 2019, 9, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.N.; Chi, H.N.Q.; Chung, D.C.; Long, L.T. Morphological changes during replicative senescence in bovine ovarian granulosa cells. Cell Cycle 2019, 18, 1490–1497. [Google Scholar] [CrossRef]
- Leguy, C.; Delfos, R.; Pourquie, M.; Poelma, C.; Krooneman, J.; Westerweel, J.; Van Loon, J. Fluid motion for microgravity simulations in a random positioning machine. Gravit. Space Biol. 2011, 25, 36–39. [Google Scholar]
- Zhang, S.; Zheng, D.; Wu, Y.; Lin, W.; Chen, Z.; Meng, L.; Liu, J.; Zhou, Y. Simulated Microgravity Using a Rotary Culture System Compromises the In Vitro Development of Mouse Preantral Follicles. PLoS ONE 2016, 11, e0151062. [Google Scholar] [CrossRef] [Green Version]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2015, 6, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell. 2019, 74, 758–770.e4. [Google Scholar] [CrossRef]
- Jha, R.; Wu, Q.; Singh, M.; Preininger, M.; Han, P.; Ding, G.; Cho, H.C.; Jo, H.; Maher, K.O.; Wagner, M.B.; et al. Simulated Microgravity and 3D Culture Enhance Induction, Viability, Proliferation and Differentiation of Cardiac Progenitors from Human Pluripotent Stem Cells. Sci. Rep. 2016, 6, 30956. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Response of SAOS-2 cells to simulated microgravity and effect of biocompatible sol–gel hybrid coatings. Acta Astronaut. 2016, 122, 237–242. [Google Scholar] [CrossRef]
- Ebnerasuly, F.; Hajebrahimi, Z.; Tabaie, S.M.; Darbouy, M. Simulated Microgravity Condition Alters the Gene Expression of some ECM and Adhesive Molecules in Adipose-Derived Stem Cells. Int. J. Mol. Cell. Med. 2018, 7, 146–157. [Google Scholar] [PubMed]
- Avitabile, E.; Fusco, L.; Minardi, S.; Orecchioni, M.; Zavan, B.; Yilmazer, A.; Rauner, M.; Pippia, P.; Tasciotti, E.; Delogu, L.G. Bioinspired Scaffold Action Under the Extreme Physiological Conditions of Simulated Space Flights: Osteogenesis Enhancing Under Microgravity. Front. Bioeng. Biotechnol. 2020, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Habela, C.W.; Sontheimer, H. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle 2007, 6, 1613–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelam, S.; Richardson, B.; Barker, R.; Udave, C.; Gilroy, S.; Cameron, M.J.; Levine, H.G.; Zhang, Y. Changes in Nuclear Shape and Gene Expression in Response to Simulated Microgravity Are LINC Complex-Dependent. Int. J. Mol. Sci. 2020, 21, 6762. [Google Scholar] [CrossRef]
- Po, A.; Giuliani, A.; Masiello, M.G.; Cucina, A.; Catizone, A.; Ricci, G.; Chiacchiarini, M.; Tafani, M.; Ferretti, E.; Bizzarri, M. Phenotypic transitions enacted by simulated microgravity do not alter coherence in gene transcription profile. NPJ Microgravity 2019, 5, 27. [Google Scholar] [CrossRef]
- Corydon, T.J.; Mann, V.; Slumstrup, L.; Kopp, S.; Sahana, J.; Askou, A.L.; Magnusson, N.E.; Echegoyen, D.; Bek, T.; Sundaresan, A.; et al. Reduced Expression of Cytoskeletal and Extracellular Matrix Genes in Human Adult Retinal Pigment Epithelium Cells Exposed to Simulated Microgravity. Cell. Physiol. Biochem. 2016, 40, 1–17. [Google Scholar] [CrossRef]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2019, 19, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Cui, Y.; Song, S.; Zhang, J.; Zhou, J.; Zhang, J. Simulated weightlessness by tail-suspension affects follicle development and reproductive capacity in rats. Int. J. Clin. Exp. Pathol. 2016, 9, 12208–12218. [Google Scholar]
- Zhang, S.; Wu, Y.; Weng, Y.; Xu, Z.; Chen, W.; Zheng, D.; Lin, W.; Liu, J.; Zhou, Y. In Vitro Growth of Mouse Preantral Follicles Under Simulated Microgravity. J. Vis. Exp. 2017, 55641. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.X.; Son, H.N.; Chi, H.N.Q.; Huy, H.N.Q.; Minh, N.T.; Tram, N.T.T.; Huyen, N.T.T.; Quan, T.M.; Chung, D.C.; Nhung, T.H.; et al. Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells. Curr. Issues Mol. Biol. 2021, 43, 2210-2219. https://doi.org/10.3390/cimb43030155
Dai TX, Son HN, Chi HNQ, Huy HNQ, Minh NT, Tram NTT, Huyen NTT, Quan TM, Chung DC, Nhung TH, et al. Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells. Current Issues in Molecular Biology. 2021; 43(3):2210-2219. https://doi.org/10.3390/cimb43030155
Chicago/Turabian StyleDai, Truong Xuan, Hoang Nghia Son, Ho Nguyen Quynh Chi, Hoang Nghia Quang Huy, Nguyen Thai Minh, Nguyen Thi Thuy Tram, Nguyen Thi Thuong Huyen, To Minh Quan, Doan Chinh Chung, Truong Hai Nhung, and et al. 2021. "Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells" Current Issues in Molecular Biology 43, no. 3: 2210-2219. https://doi.org/10.3390/cimb43030155
APA StyleDai, T. X., Son, H. N., Chi, H. N. Q., Huy, H. N. Q., Minh, N. T., Tram, N. T. T., Huyen, N. T. T., Quan, T. M., Chung, D. C., Nhung, T. H., Minh, T. T., Diem, T. H., Mai, N. T. P., & Long, L. T. (2021). Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells. Current Issues in Molecular Biology, 43(3), 2210-2219. https://doi.org/10.3390/cimb43030155






