Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. 4T1 Cells
2.3. Fibroblasts
2.4. Activation of Fibroblasts
2.5. Transplantation of 4T1 Cells
2.6. Activation of Macrophages by Necrotic Cells
2.7. Quantification of MCP-1 Concentration
2.8. Western Blotting
2.9. qRT-PCR
2.10. RT-PCR
2.11. Immunohistochemistry
2.12. In Situ Hybridization (ISH)
2.13. Statistical Analysis
3. Results
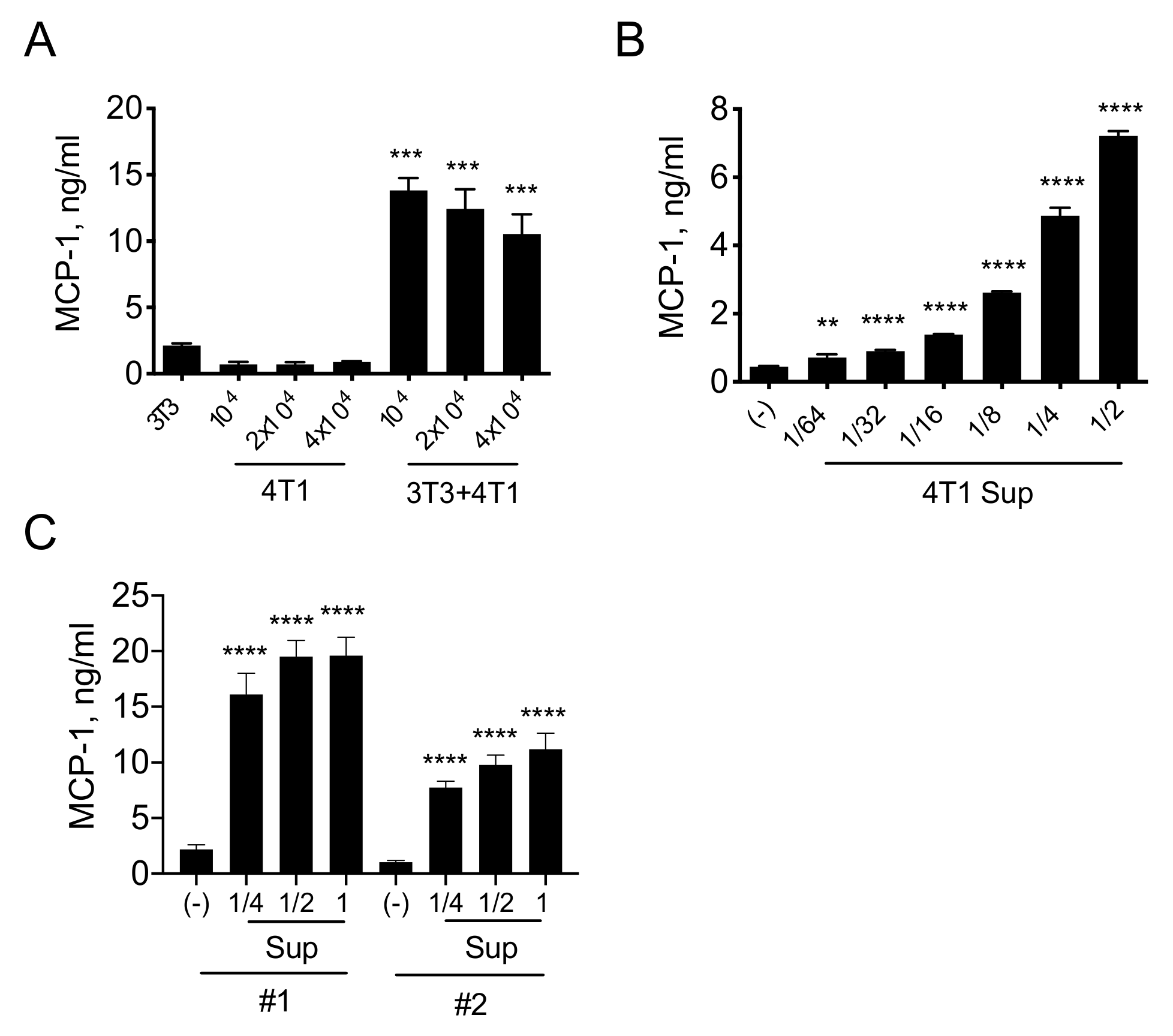
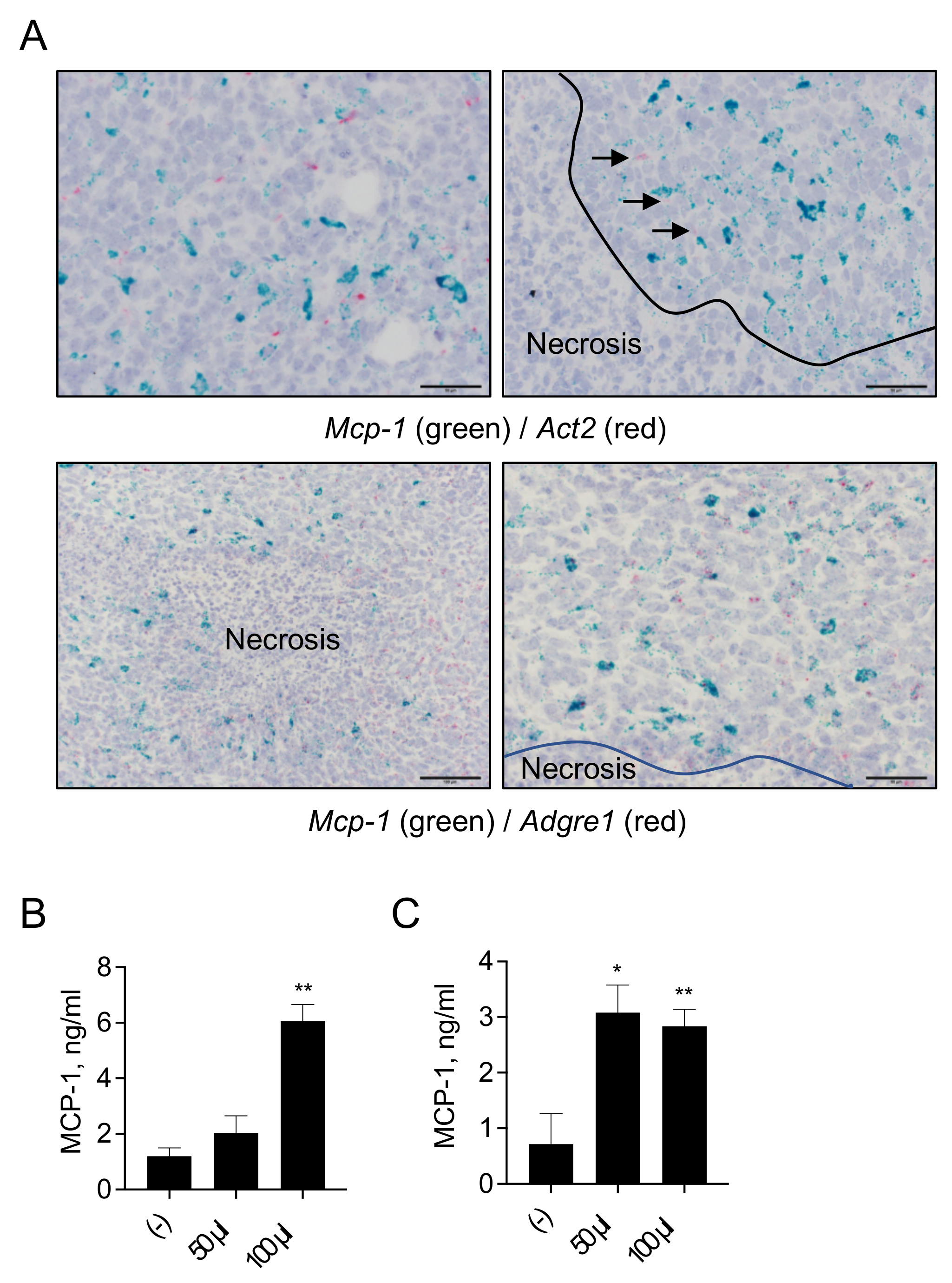
3.1. A Product(s) of 4T1 Cells Induces MCP-1 Production by Fibroblasts
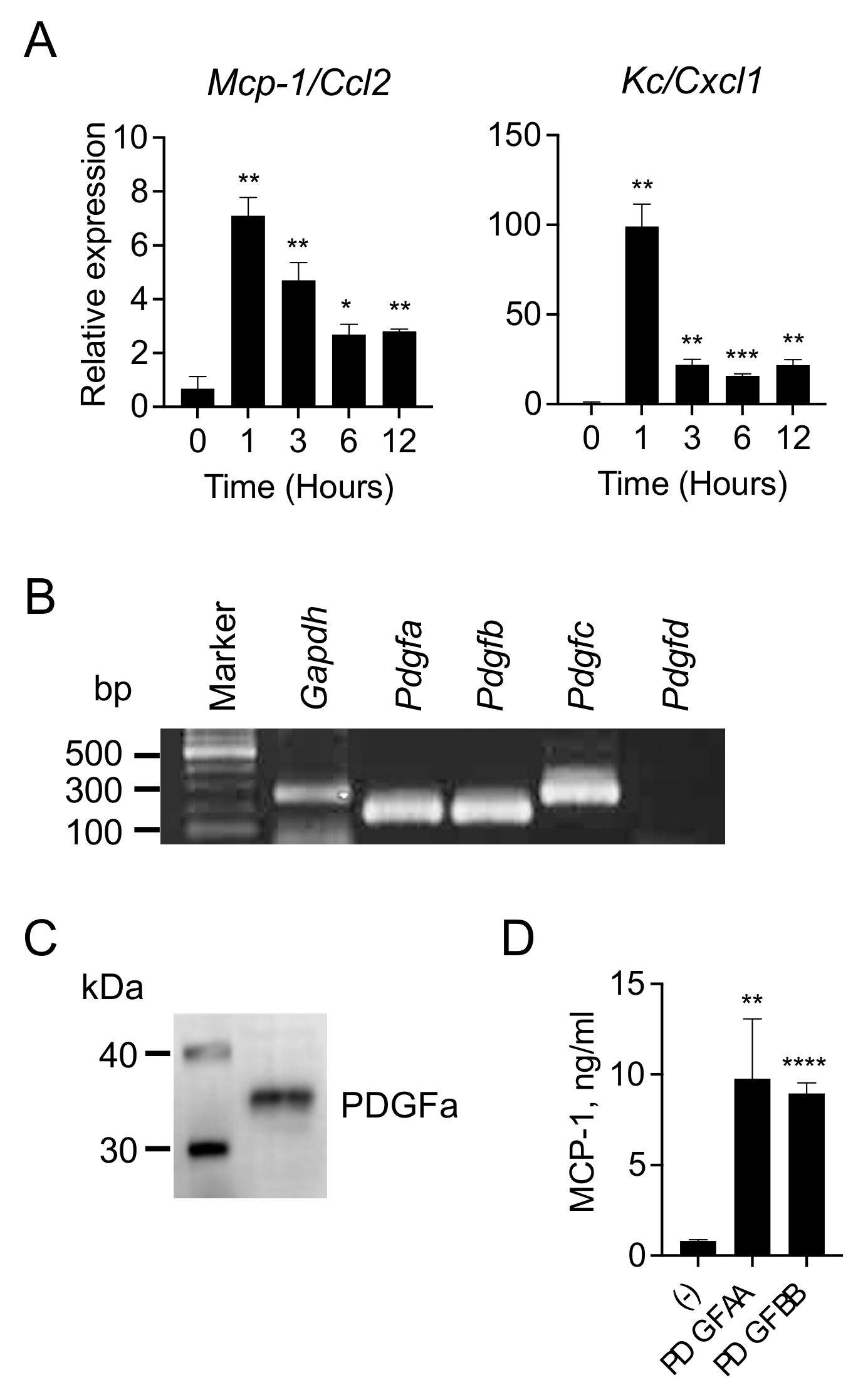
3.2. 4T1 Cells Produce and Release PDGFs
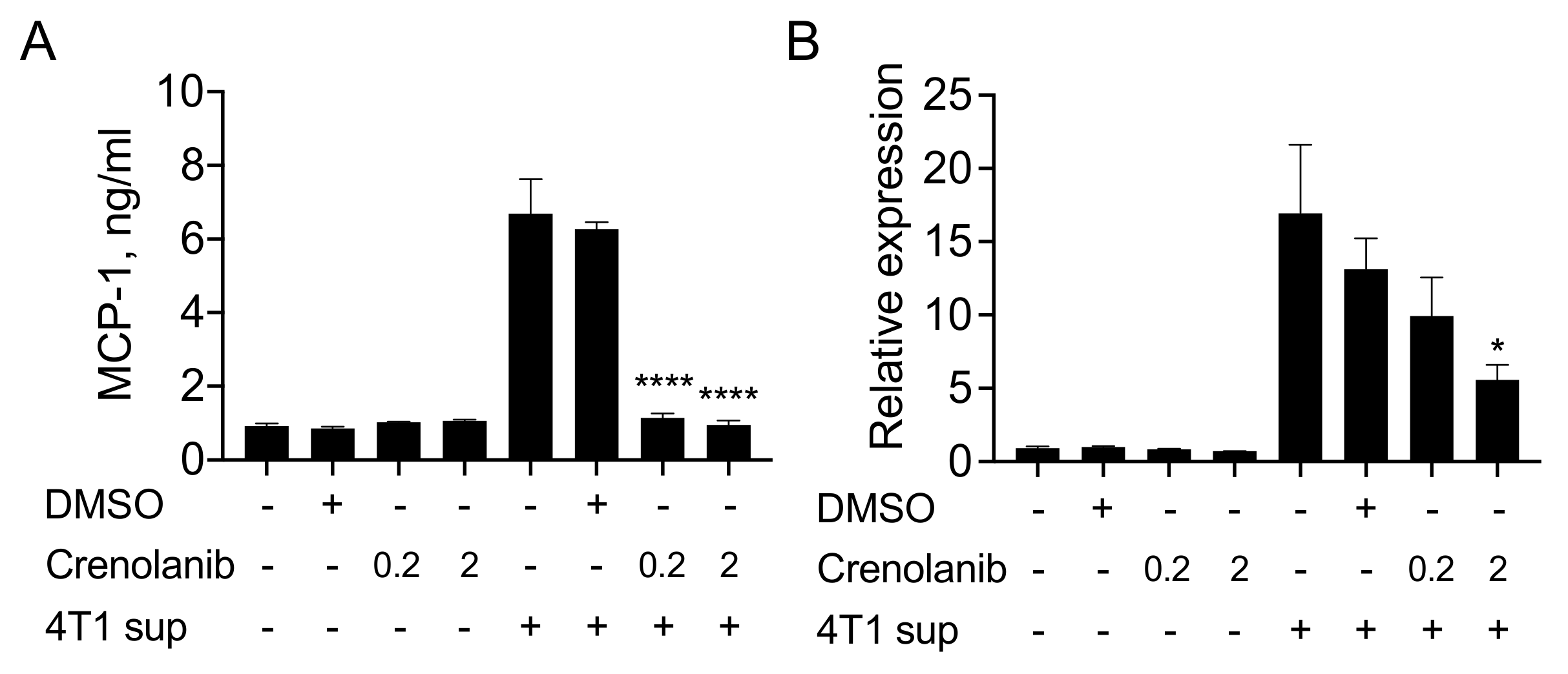
3.3. The PDGF Receptor Inhibitor Crenolanib Inhibits 4T1 Cell-Induced MCP-1 Production by Fibroblasts
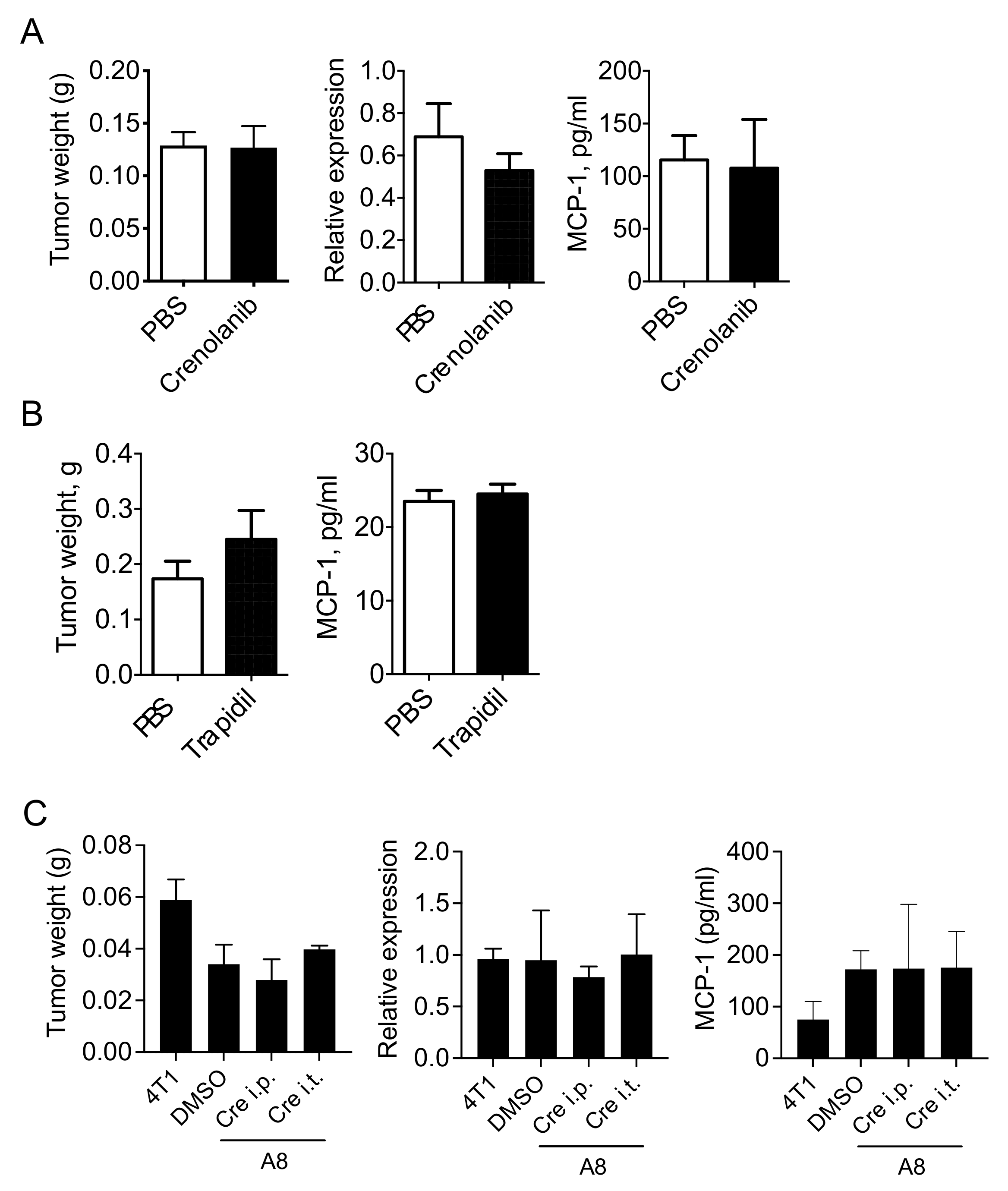
3.4. PDGFR Antagonists Do Not Reduce the Level of MCP-1 in 4T1 Tumor-Bearing Mice
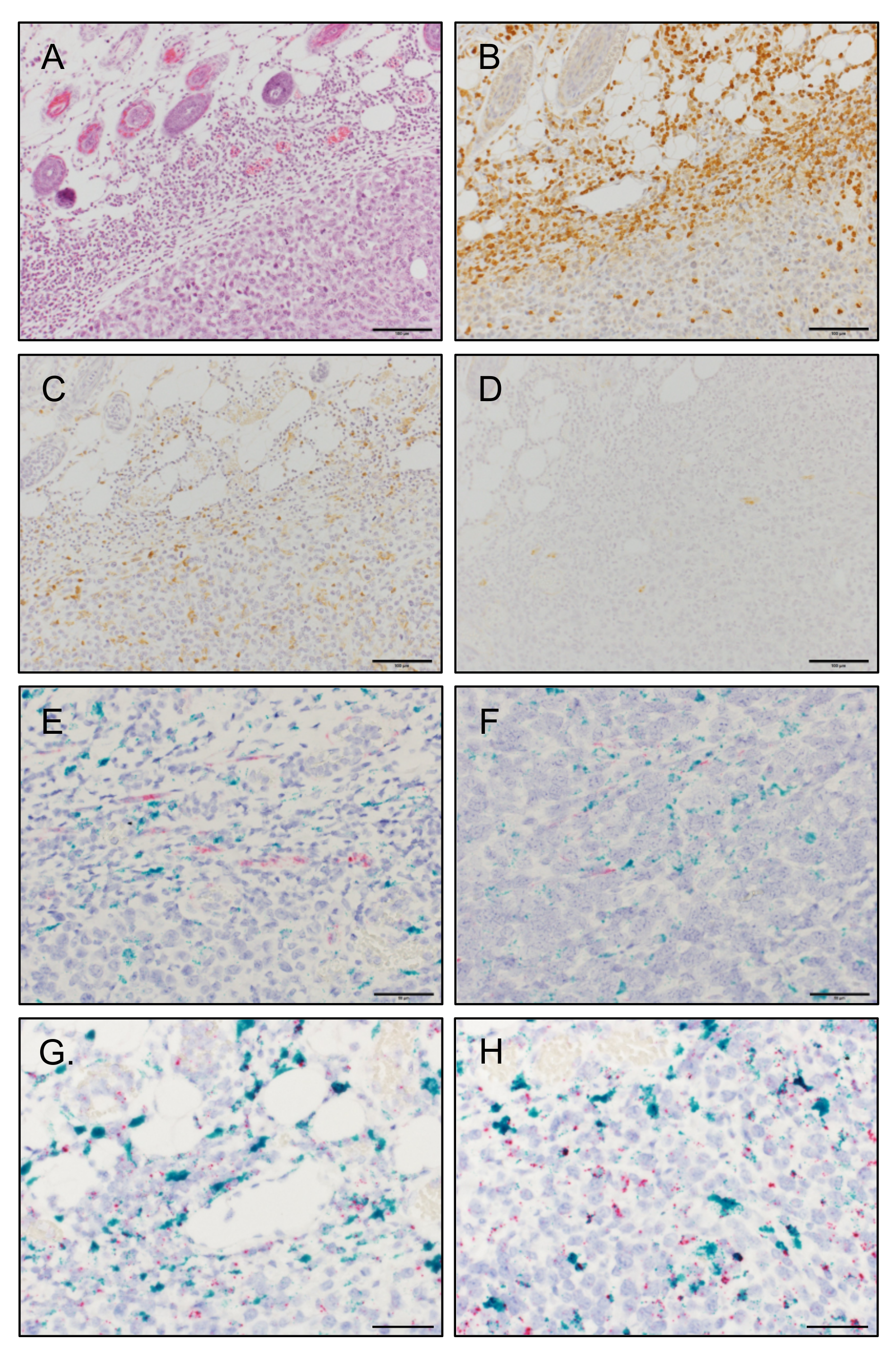
3.5. MCP-1 Is Mainly Expressed by F4/80+ Macrophages, but Not by Fibroblasts, in 4T1 Tumors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef] [Green Version]
- Dexter, D.L.; Kowalski, H.M.; Blazar, B.A.; Fligiel, Z.; Vogel, R.; Heppner, G.H. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978, 38, 3174–3181. [Google Scholar]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc Immunol. 2001, 39, 20.2.1–20.2.16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenblast, E.; Soto, M.; Gutiérrez-Ángel, S.; Hartl, C.A.; Gable, A.L.; Maceli, A.R.; Erard, N.; Williams, A.M.; Kim, S.Y.; Dickopf, S.; et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 2015, 520, 358–362. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally. Cell Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Valković, T.; Lucin, K.; Krstulja, M.; Dobi-Babić, R.; Jonjić, N. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res. Pract. 1998, 194, 335–340. [Google Scholar] [CrossRef]
- Saji, H.; Koike, M.; Yamori, T.; Saji, S.; Seiki, M.; Matsushima, K.; Toi, M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 2001, 92, 1085–1091. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sangai, T.; Ishii, G.; Ikehara, A.; Nagashima, T.; Miyazaki, M.; Ochiai, A. Stromal MCP-1 in mammary tumors induces tumo-associated macrophage infiltration and contributes to tumor progression. Int. J. Cancer. 2009, 125, 1276–1284. [Google Scholar] [CrossRef]
- Li, D.; Ji, H.; Niu, X.; Yin, L.; Wang, Y.; Gu, Y.; Wang, J.; Zhou, X.; Zhang, H.; Zhang, Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020, 111, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Howard, O.M.Z.; Ito, T.; Kuwabara, M.; Matsukawa, A.; Chen, K.; Liu, Y.; Liu, M.; Oppenheim, J.J.; Wang, J.M. Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PLoS ONE 2013, 8, e58791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Nakamura, K.; Li, C.; Fujisawa, M.; Shiina, T.; Imamura, M.; Li, T.; Mukaida, N.; Matsukawa, A. Cancer Cell-Derived Granulocyte-Macrophage Colony-Stimulating Factor Is Dispensable for the Progression of 4T1 Murine Breast Cancer. Int. J. Mol. Sci. 2019, 20, 6342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Rolfe, M.W.; Kunkel, S.L.; Standiford, T.J.; Chensue, S.W.; Allen, R.M.; Evanoff, H.L.; Phan, S.H.; Strieter, R.M. Pulmonary fibroblast expression of interleukin-8: A model for alveolar macrophage-derived cytokine networking. Am. J. Respir. Cell Mol. Biol. 1991, 5, 493–501. [Google Scholar] [CrossRef]
- Wilborn, J.; Crofford, L.J.; Burdick, M.D.; Kunkel, S.L.; Strieter, R.M.; Peters-Golden, M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J. Clin. Invest. 1995, 95, 1861–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran, B.H.; Reffel, A.C.; Stiles, C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell 1983, 33, 939–947. [Google Scholar] [CrossRef]
- Yoshimura, T.; Leonard, E.J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J. Immunol. 1990, 144, 2377–2383. [Google Scholar]
- Rollins, B.J. JE/MCP-1: An early-response gene encodes a monocyte-specific cytokine. Cancer Cells. 1991, 3, 517–524. [Google Scholar]
- Bronzert, D.A.; Pantazis, P.; Antoniades, H.N.; Kasid, A.; Davidson, N.; Dickson, R.B.; Lippman, M.E. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc. Natl. Acad. Sci. USA 1987, 84, 5763–5767. [Google Scholar] [CrossRef] [Green Version]
- Demoulin, J.B.; Montano-Almendras, C.P. Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am. J. Blood Res. 2012, 2, 44–56. [Google Scholar]
- Lewis, N.L.; Lewis, L.D.; Eder, J.P.; Reddy, N.J.; Guo, F.; Pierce, K.J.; Olszanski, A.J.; Cohen, R.B. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J. Clin. Oncol. 2009, 27, 5262–5269. [Google Scholar] [CrossRef]
- Smith, C.C.; Lasater, E.A.; Lin, K.C.; Wang, Q.; McCreery, M.Q.; Stewart, W.K.; Damon, L.E.; Perl, A.E.; Jeschke, G.R.; Sugita, M.; et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc. Natl. Acad. Sci. USA 2014, 111, 5319–5324. [Google Scholar] [CrossRef] [Green Version]
- Galanis, A.; Ma, H.; Rajkhowa, T.; Ramachandran, A.; Small, D.; Cortes, J.; Levis, M. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring poInt. mutants. Blood 2014, 123, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Bailey, E.; Nguyen, B.; Yang, X.; Piloto, O.; Levis, M.; Small, D. Further activation of FLT3 mutants by FLT3 ligand. Oncogene 2011, 30, 4004–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, D.; Adolph, L.; Köhler, J.P.; Buschmann, T.; Luedde, T.; Häussinger, D.; Kordes, C. Improved Recovery from Liver Fibrosis by Crenolanib. Cells 2021, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Berndsen, R.H.; Castrogiovanni, C.; Weiss, A.; Rausch, M.; Dallinga, M.G.; Miljkovic-Licina, M.; Klaassen, I.; Meraldi, P.; van Beijnum, J.R.; Nowak-Sliwinska, P. Anti-angiogenic effects of crenolanib are mediated by mitotic modulation independently of PDGFR expression. Br. J. Cancer 2019, 121, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Bender, L.H.; Abbate, F.; Walters, I.B. Intratumoral administration of a novel cytotoxic formulation with strong tissue dispersive properties regresses tumor growth and elicits systemic adaptive immunity in in vivo models. Int. J. Mol. Sci. 2020, 21, 4493. [Google Scholar] [CrossRef]
- Ohnishi, H.; Yamaguchi, K.; Shimada, S.; Suzuki, Y.; Kumagai, A. A new approach to the treatment of atherosclerosis ad trapidil as an antagonist to platelet-derived growth factor. Life Sci. 1981, 28, 1641–1646. [Google Scholar] [CrossRef]
- Deguchi, J.; Abe, J.; Makuuchi, M.; Takuwa, Y. Inhibitory effects of trapidil on PDGF signaling in balloon-injured rat carotid artery. Life Sci. 1999, 65, 2791–2799. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, H.N.; Lee, J.H.; Jin, W.J.; Hwang, S.J.; Kim, H.H.; Ha, H.; Lee, Z.H. Trapidil, a platelet-derived growth factor antagonist, inhibits osteoclastogenesis by down-regulating NFATc1 and suppresses bone loss in mice. Biochem. Pharmacol. 2013, 86, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, I.M.; Grill, A.; Fremter, K.; Steppan, D.; Hellmuth, M.K.; Castrop, H. Renal Interstitial Platelet-Derived Growth Factor Receptor-β Cells Support Proximal Tubular Regeneration. J. Am. Soc. Nephrol. 2018, 29, 1383–1396. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Sengupta, K.; Dhar, K.; Mehta, S.; D’Amore, P.A.; Dhar, G.; Banerjee, S.K. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol. Carcinog. 2006, 45, 871–880. [Google Scholar] [CrossRef]
- Jitariu, A.A.; Raica, M.; Cîmpean, A.M.; Suciu, S.C. The role of PDGF-B/PDGFR-BETA axis in the normal development and carcinogenesis of the breast. Crit. Rev. Oncol Hematol. 2018, 131, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Ewens, A.; Mihich, E.; Ehrke, M.J. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer Res. 2005, 25, 3905–3916. [Google Scholar] [PubMed]
- Usary, J.; Darr, D.B.; Pfefferle, A.; Perou, C.M. Genetically Engineered Mouse Models of Distinct Breast Cancer Subtypes. Curr. Protoc. Pharmacol. 2017, 72, 14.38.1–14.38.11. [Google Scholar] [CrossRef] [Green Version]
- Tsuyada, A.; Wang, S.E. Fibroblast-derived CCL2 induces cancer stem cells response. Cancer Res. 2013, 73, 1032–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran-Struuck, R.; Dysko, R.C. Principles of bone marrow transplantation (BMT): Providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 11–22. [Google Scholar] [PubMed]
- Patel, S. Danger-Associated Molecular Patterns (DAMPs): The Derivatives and Triggers of Inflammation. Curr. Allergy Asthma Rep. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, M.; Li, T.; Li, C.; Fujisawa, M.; Mukaida, N.; Matsukawa, A.; Yoshimura, T. Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer. Curr. Issues Mol. Biol. 2021, 43, 1726-1740. https://doi.org/10.3390/cimb43030122
Imamura M, Li T, Li C, Fujisawa M, Mukaida N, Matsukawa A, Yoshimura T. Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer. Current Issues in Molecular Biology. 2021; 43(3):1726-1740. https://doi.org/10.3390/cimb43030122
Chicago/Turabian StyleImamura, Mayu, Tiantian Li, Chunning Li, Masayoshi Fujisawa, Naofumi Mukaida, Akihiro Matsukawa, and Teizo Yoshimura. 2021. "Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer" Current Issues in Molecular Biology 43, no. 3: 1726-1740. https://doi.org/10.3390/cimb43030122
APA StyleImamura, M., Li, T., Li, C., Fujisawa, M., Mukaida, N., Matsukawa, A., & Yoshimura, T. (2021). Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer. Current Issues in Molecular Biology, 43(3), 1726-1740. https://doi.org/10.3390/cimb43030122





