Mycorrhizal Patterns in the Roots of Dominant Festuca rubra in a High-Natural-Value Grassland
Abstract
:1. Introduction
- (i).
- Is the colonization frequency a parameter for defining the primary permissiveness of the root toward the symbiont?
- (ii).
- Does the intensity of colonization define the secondary permissiveness of the root toward the symbiont?
- (iii).
- Is the intensity of colonization dependent on frequency?
- (iv).
- Is mycorrhizal colonization a stable mechanism, or does it have fluctuations in the root?
- (v).
- Is the mycorrhizal pattern linear or fluctuating, and how sensitive is the change?
- (vi).
- Is the complete exploration of colonization data and maps efficient in identifying colonization strategies?
- (vii).
- Is the assessment of the colonization dynamics by the MycoPatt tool effective in the real positioning of the symbiont in the root cortex?
2. Results
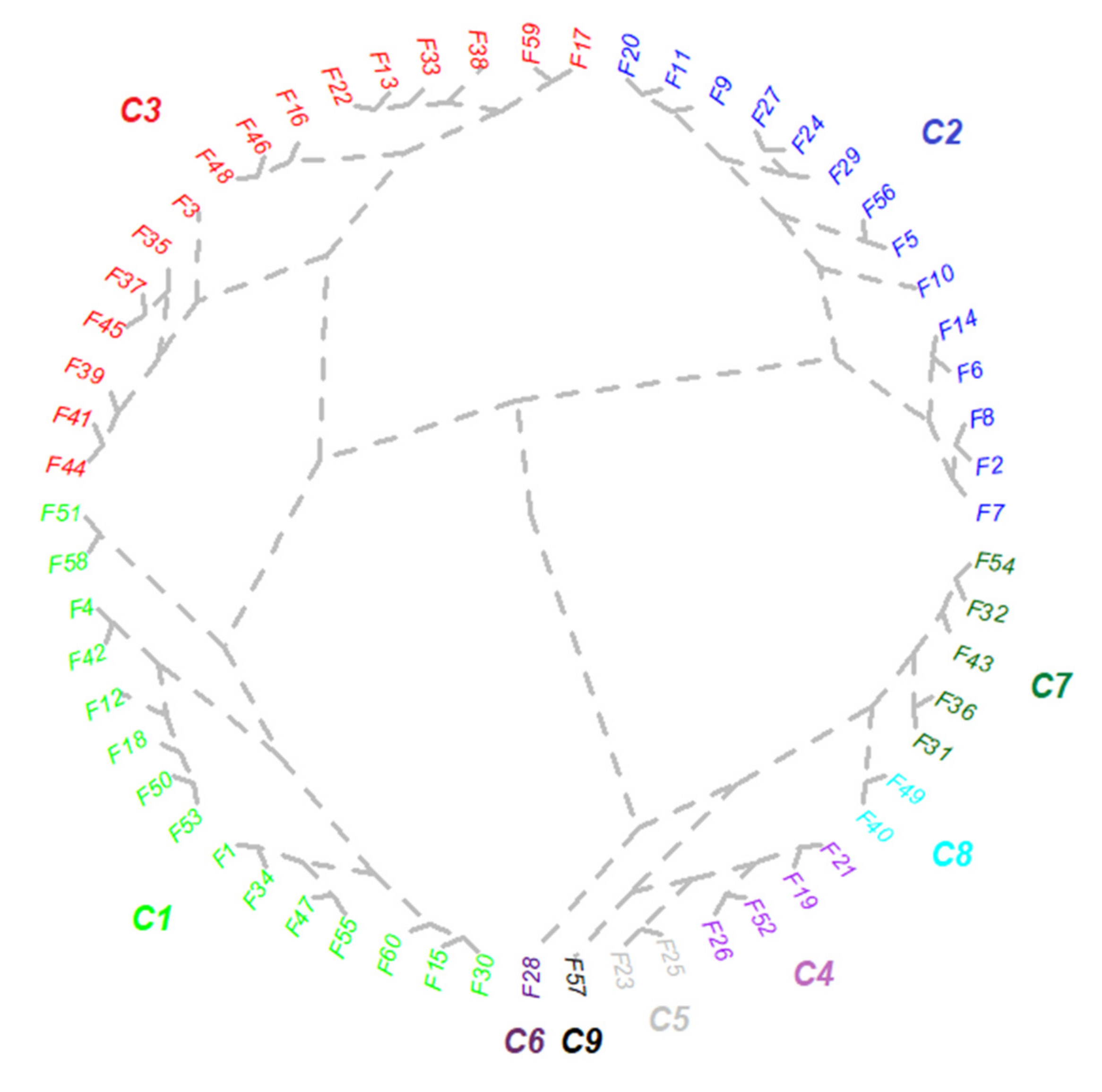
2.1. Establishment of Best-Class Solution for Grouping Mycorrhizal Parameters
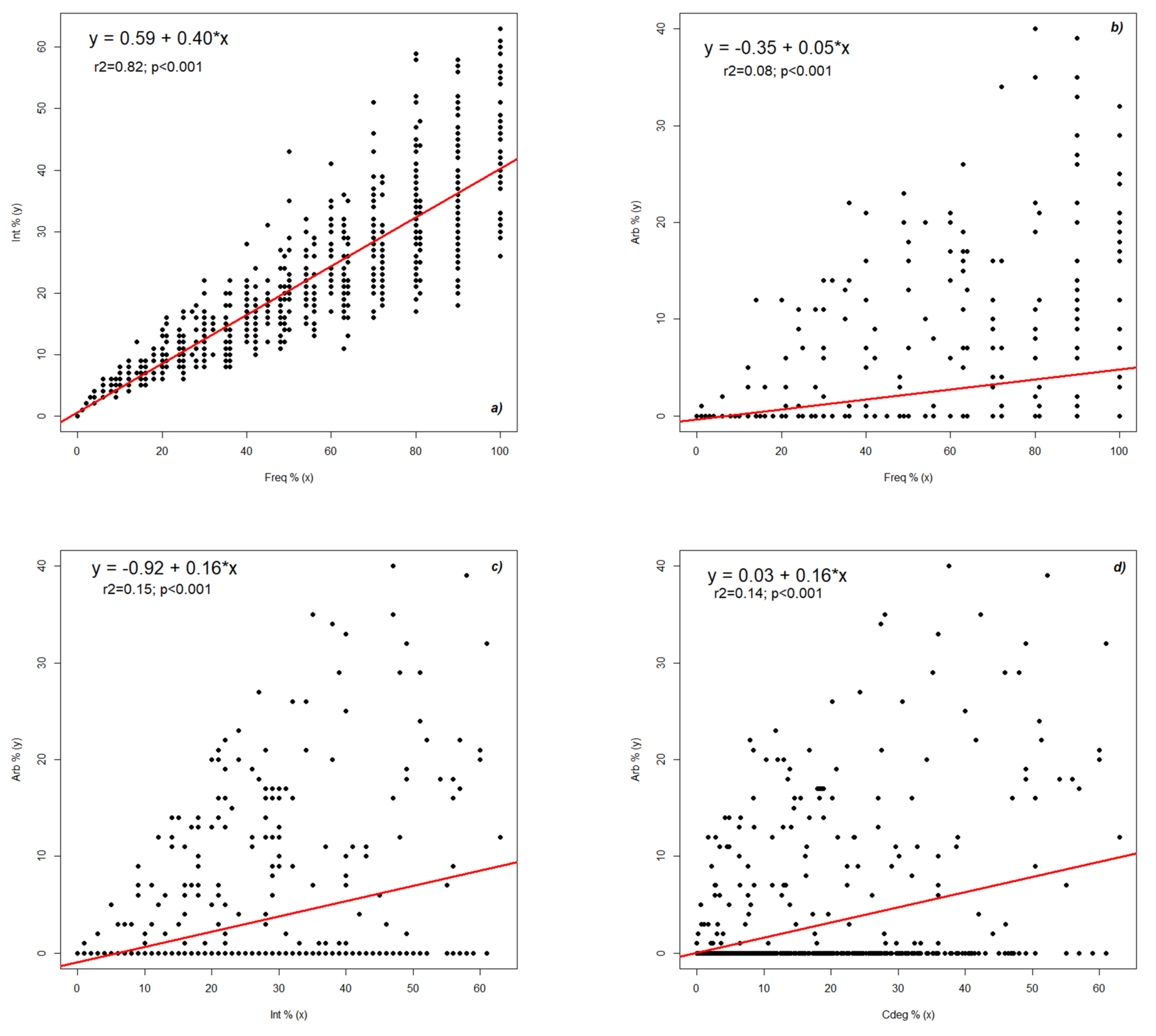
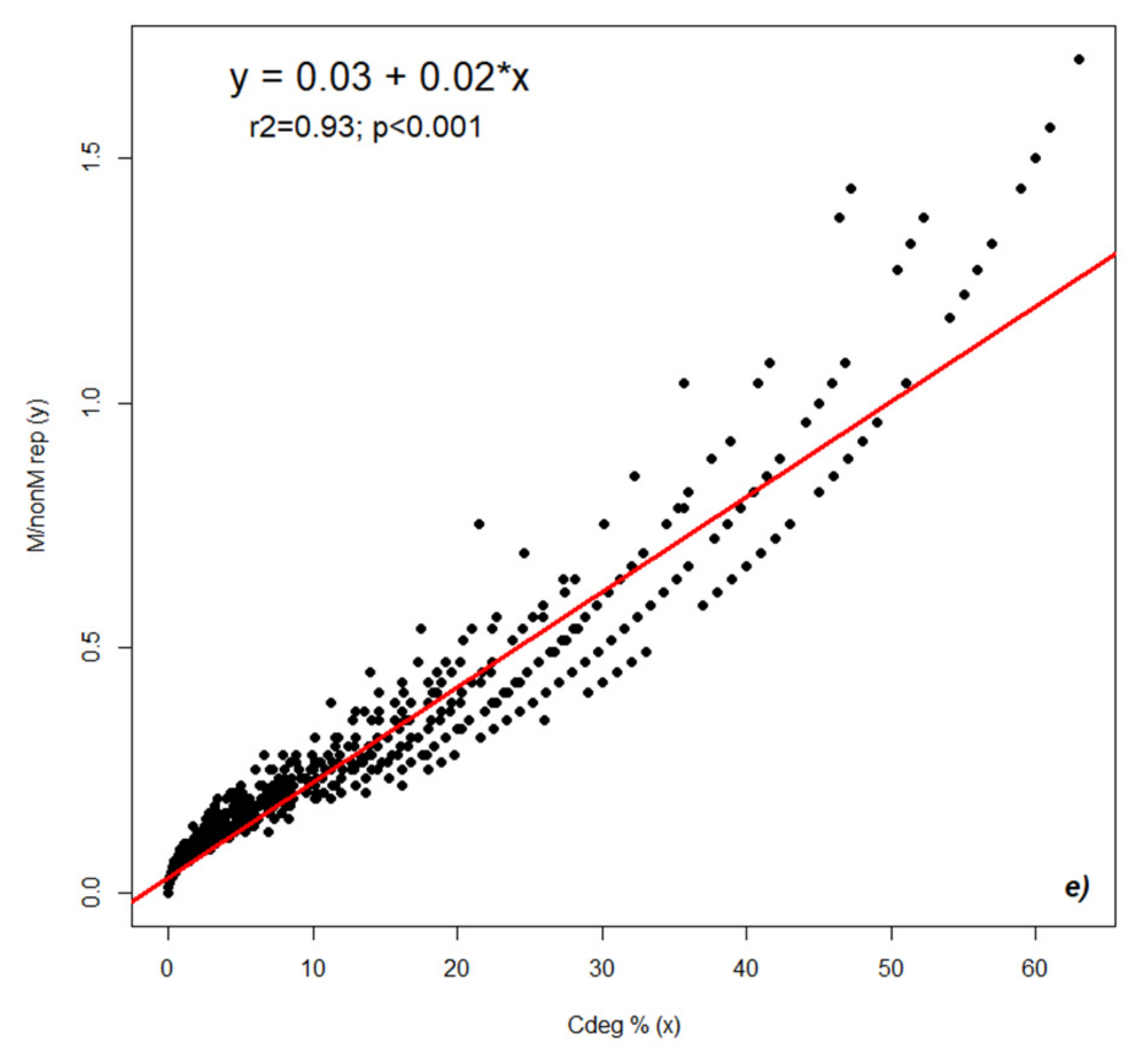
2.2. Dynamics of Colonization Parameters
2.3. Analysis and Forecast of the Colonization Development in the Root Cortex
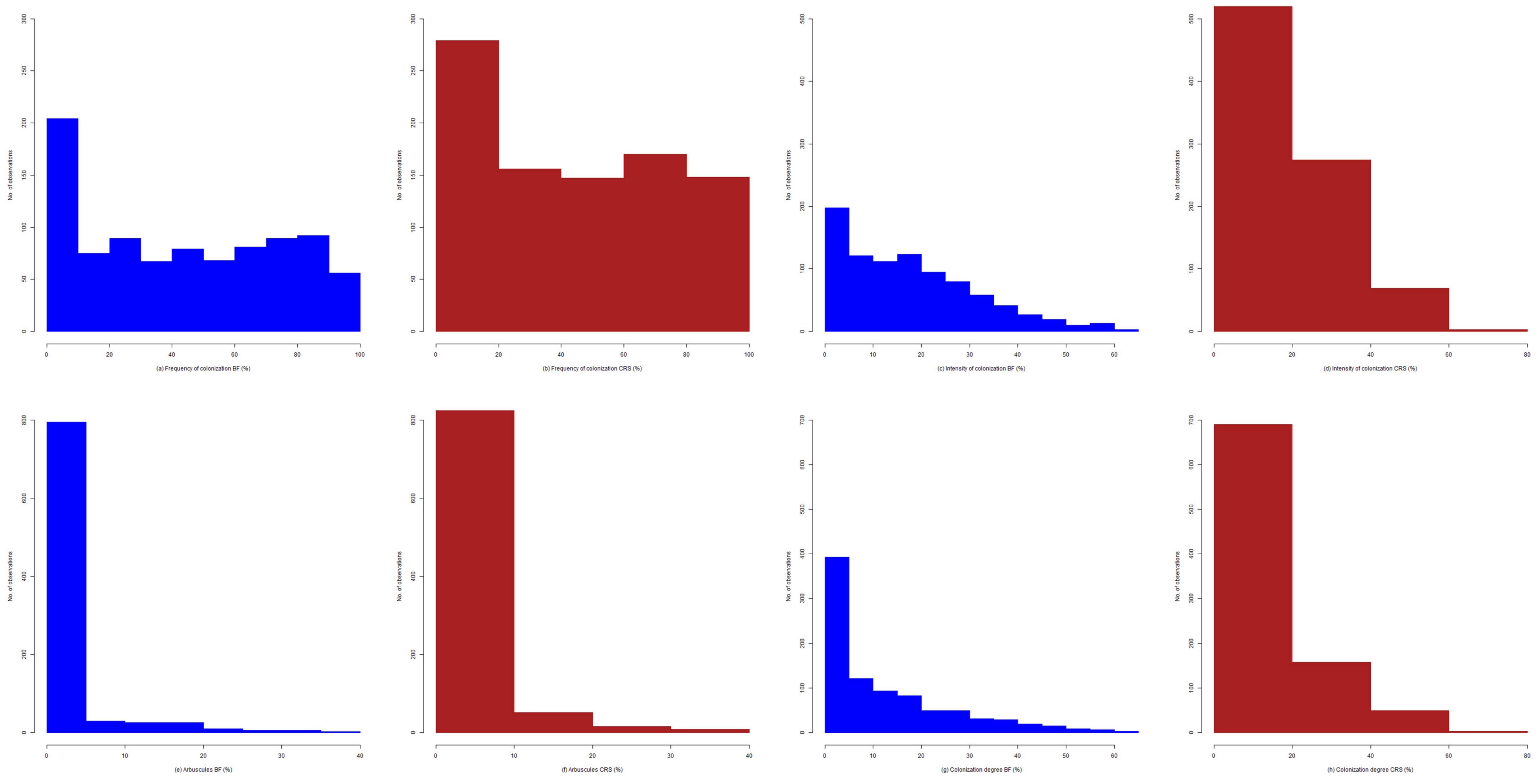
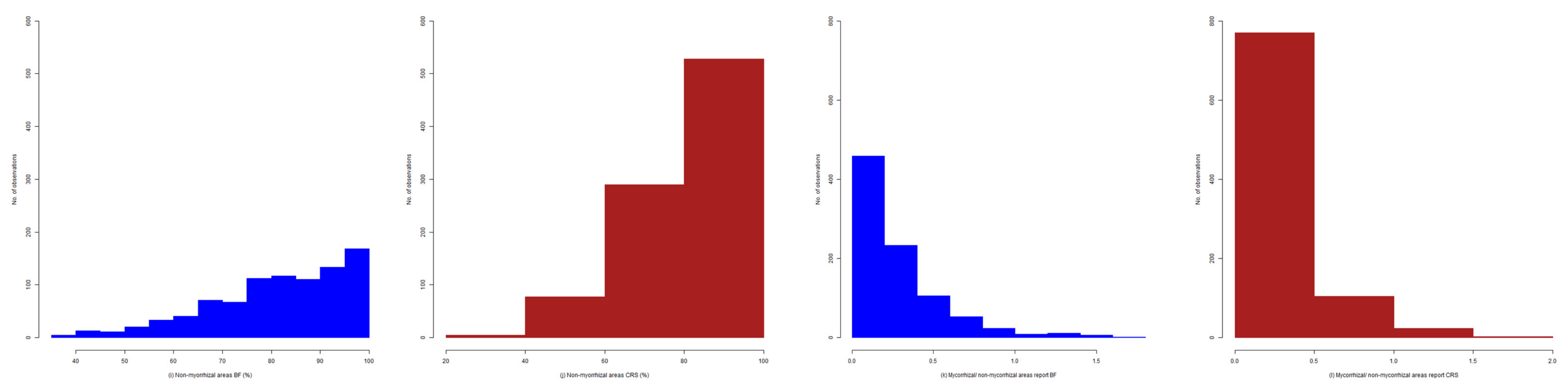
2.4. Exploratory Analysis of Root Colonization in Festuca rubra
2.5. Colonization Strategy in Festuca rubra
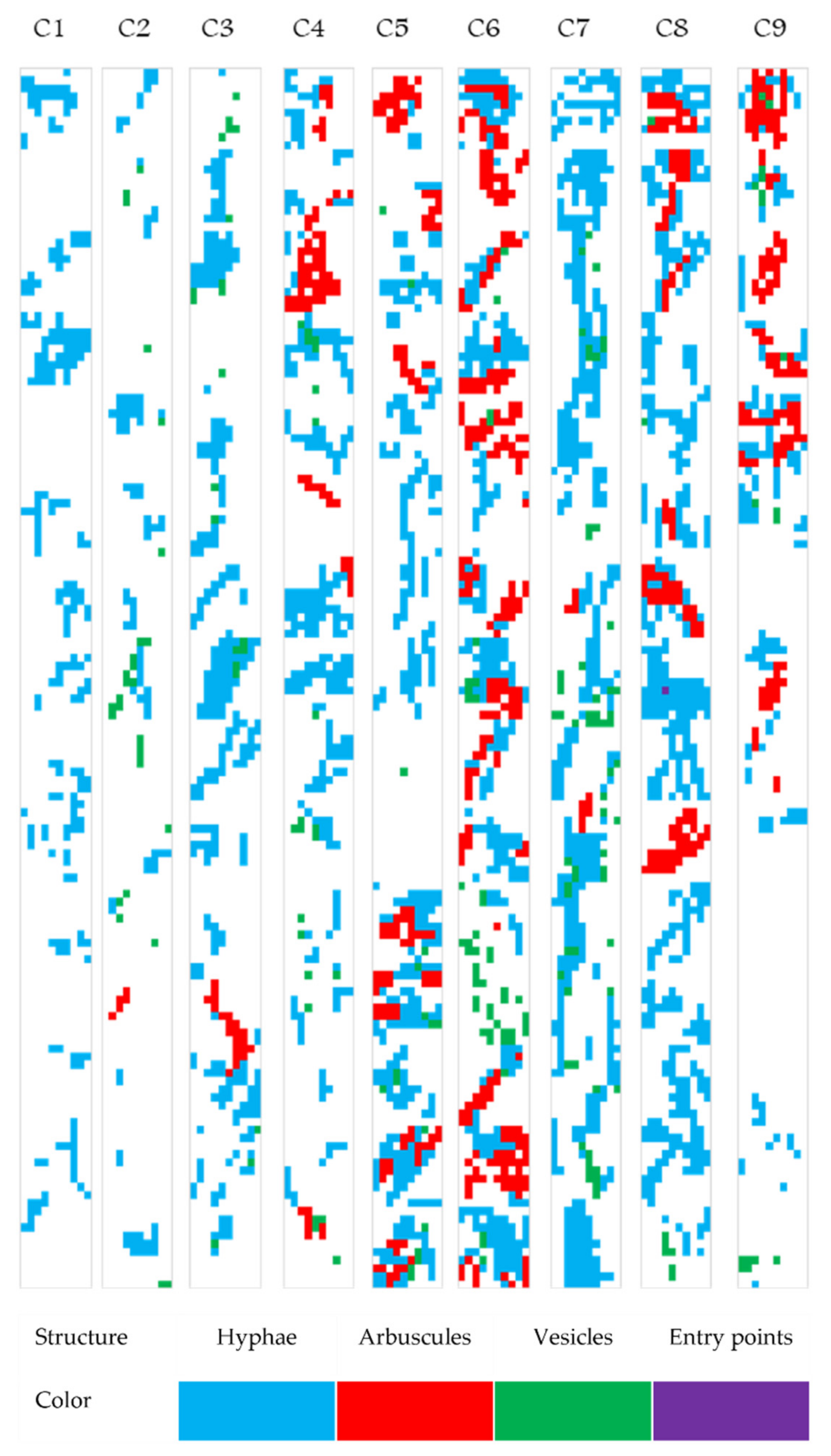
2.6. Mapping the Native Mycorrhizal Patterns in Festuca rubra
3. Discussion
4. Conclusions
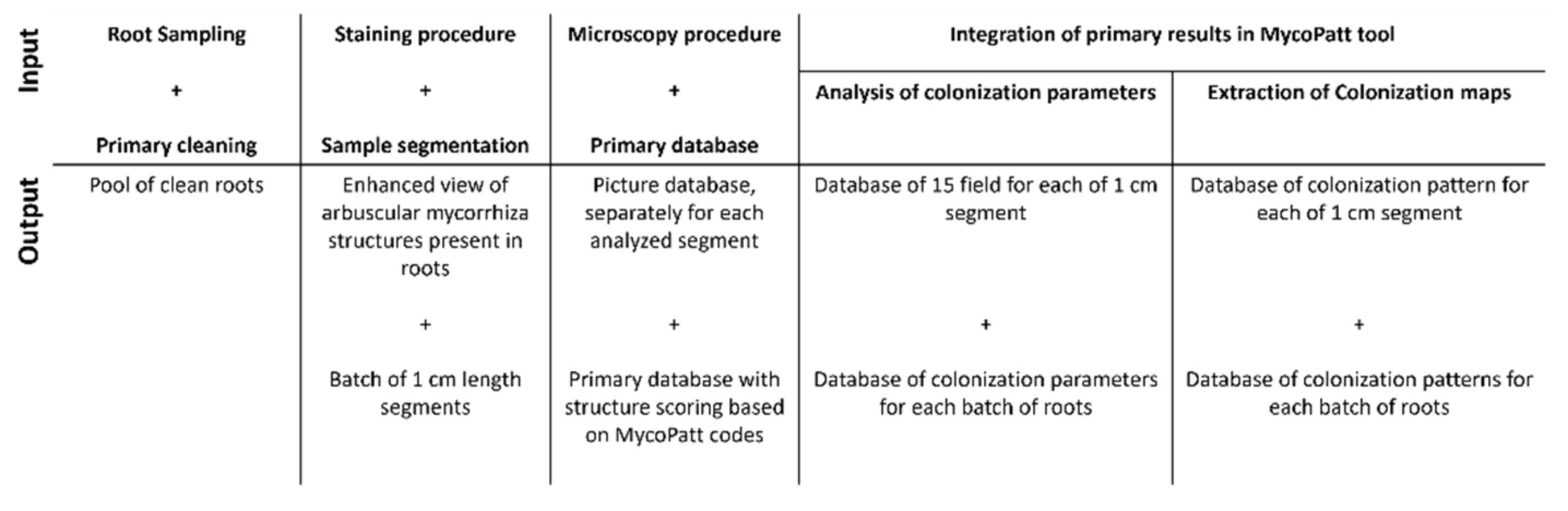
5. Materials and Methods
5.1. Experimental Field and Biological Material
5.2. Laboratory Techniques for Mycorrhizal Analysis
5.3. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squires, V.R.; Dengler, J.; Hua, L.; Feng, H. Grasslands of the World: Diversity, Management and Conservation; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-65220-9. [Google Scholar]
- Liu, H.; Hart, M.; Kong, Z. The distribution of arbuscular mycorrhizal fungal communities at soil aggregate level in subtropical grasslands. Arch. Agron. Soil Sci. 2021, 1–13. [Google Scholar] [CrossRef]
- Rosen, C. (Ed.) World Resources 2000–2001: People and Ecosystems: The Fraying Web of Life; Elsevier: Amsterdam, The Netherlands, 2000; Available online: https://www.wri.org/research/world-resources-2000–2001 (accessed on 15 November 2021).
- Gibson, D.J. Grasses and Grassland Ecology; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; ISBN 978-0-19-852919-4. [Google Scholar]
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of Palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Poeplau, C. Grassland soil organic carbon stocks along management intensity and warming gradients. Grass Forage Sci. 2021, 76, 186–195. [Google Scholar] [CrossRef]
- Dominati, E.; Mackay, A.; Green, S.; Patterson, M. A soil change-based methodology for the quantification and valuation of ecosystem services from agro-ecosystems: A case study of pastoral agriculture in New Zealand. Ecol. Econ. 2014, 100, 119–129. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-Based Inoculants: Role in Next Green Revolution. In Environmental Concerns and Sustainable Development: Volume 2: Biodiversity, Soil and Waste Management; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 191–246. ISBN 9789811363580. [Google Scholar]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2018, 129, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, E.; Gamper, H.; Ciccolini, V.; Ercoli, L. Forage Rotations Conserve Diversity of Arbuscular Mycorrhizal Fungi and Soil Fertility. Front. Microbiol. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Xiang, A.; Qureshi, S.; Li, T.; Song, D.; Zhang, C. Quantifying the human disturbance intensity of ecosystems and its natural and socioeconomic driving factors in urban agglomeration in South China. Environ. Sci. Pollut. Res. 2021, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Šmilauer, P.; Košnar, J.; Kotilínek, M.; Pecháčková, S.; Šmilauerová, M. Host age and surrounding vegetation affect the community and colonization rates of arbuscular mycorrhizal fungi in a temperate grassland. New Phytol. 2021, 232, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A. Earthworm Ecology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdán, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms Building Up Soil Microbiota, a Review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Jamir, E.; Kangabam, R.D.; Borah, K.; Tamuly, A.; Deka Boruah, H.P.; Silla, Y. Role of Soil Microbiome and Enzyme Activi-ties in Plant Growth Nutrition and Ecological Restoration of Soil Health. In Microbes and Enzymes in Soil Health and Bioremediation; Microorganisms for Sustainability; Kumar, A., Sharma, S., Eds.; Springer: Singapore, 2019; pp. 99–132. ISBN 9789811391170. [Google Scholar]
- Zhang, L.; Hu, H.; Shen, J.-P.; He, J.-Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2011, 6, 1032–1045. [Google Scholar] [CrossRef] [Green Version]
- Carlier, L.; Rotar, I.; Vlahova, M.; Vidican, R. Importance and Functions of Grasslands. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 25–30. [Google Scholar] [CrossRef]
- Vezzani, F.; Anderson, C.; Meenken, E.; Gillespie, R.; Peterson, M.; Beare, M.H. The importance of plants to development and maintenance of soil structure, microbial communities and ecosystem functions. Soil Tillage Res. 2017, 175, 139–149. [Google Scholar] [CrossRef]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. Structural diversity in (vesicular)–arbuscular mycorrhizal symbioses. New Phytol. 1997, 137, 373–388. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-08-055934-6. [Google Scholar]
- Henry, H.A.; Gibson, D.J.; Newman, J.A. Biogeochemical cycling in grasslands under climate change. Grassl. Clim. Change 2019, 1, 115. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root traits explain rhizosphere fungal community composition among temperate grassland plant species. New Phytol. 2021, 229, 1492–1507. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Helgason, T.; Feng, H.; Sherlock, D.J.; Young, J.P.W.; Fitter, A.H. Arbuscular mycorrhizal communities associated with maples (Acer spp.) in a common garden are influenced by season and host plant. Botany 2014, 92, 321–326. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology 2010, 91, 1163–1171. [Google Scholar] [CrossRef]
- Păcurar, F.; Rotar, I. The Effect of Low-Input upon Semi-Natural Grasslands of Apuseni Mountains. Agron. Ser. Sci. Res./Lucr. Stiintifice Ser. Agron. 2011, 54, 263–266. [Google Scholar]
- Řezáčová, V.; Konvalinková, T.; Jansa, J. Carbon Fluxes in Mycorrhizal Plants. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–21. ISBN 978-3-319-57849-1. [Google Scholar]
- Stoian, V.; Vidican, R.; Crişan, I.; Puia, C.; Şandor, M.; Stoian, V.A.; Păcurar, F.; Vaida, I. Sensitive approach and future perspectives in microscopic patterns of mycorrhizal roots. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Skálová, H.; Vosatka, M. Growth response of threeFestuca rubra clones to light quality and arbuscular mycorrhiza. Folia Geobot. Phytotaxon. 1998, 33, 159–169. [Google Scholar] [CrossRef]
- Yang, G.; Wagg, C.; Veresoglou, S.; Hempel, S.; Rillig, M. How Soil Biota Drive Ecosystem Stability. Trends Plant Sci. 2018, 23, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Mardhiah, U.; Caruso, T.; Gurnell, A.; Rillig, M. Arbuscular mycorrhizal fungal hyphae reduce soil erosion by surface water flow in a greenhouse experiment. Appl. Soil Ecol. 2016, 99, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Dickie, I.A. Invasive belowground mutualists of woody plants. Biol. Invasions 2014, 16, 645–661. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bennett, B.M.; Burrows, L.E.; Nuñez, M.A.; Peltzer, D.A.; Porté, A.; Richardson, D.M.; Rejmánek, M.; Rundel, P.W.; van Wilgen, B.W. Conflicting values: Ecosystem services and invasive tree management. Biol. Invasions 2013, 16, 705–719. [Google Scholar] [CrossRef]
- Vázquez-De-Aldana, B.R.; Zabalgogeazcoa, I.; García-Ciudad, A.; García-Criado, B. An Epichloë endophyte affects the competitive ability of Festuca rubra against other grassland species. Plant Soil 2012, 362, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, M.G.A.; De Bruin, S.; Luckerhoff, L.; van Logtestijn, R.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2015, 10, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Bunn, R.A.; Lekberg, Y.; Gallagher, C.; Rosendahl, S.; Ramsey, P.W. Grassland invaders and their mycorrhizal symbionts: A study across climate and invasion gradients. Ecol. Evol. 2014, 4, 794–805. [Google Scholar] [CrossRef]
- Kobayashi, D.Y.; Crouch, J.A. Bacterial/Fungal Interactions: From Pathogens to Mutualistic Endosymbionts. Annu. Rev. Phytopathol. 2009, 47, 63–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnoor, T.K.; Mårtensson, L.-M.D.; Olsson, P.A. Soil disturbance alters plant community composition and decreases mycorrhizal carbon allocation in a sandy grassland. Oecologia 2011, 167, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Asghari, H.R.; Cavagnaro, T.R. Arbuscular Mycorrhizas Reduce Nitrogen Loss via Leaching. PLoS ONE 2012, 7, e29825. [Google Scholar] [CrossRef] [Green Version]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Andersen, T. Effects of Root Zone Composition and Nitrogen and Phosphorus Rates on Mycorrhizal Colonization in Different Turfgrass Species on Sand-Based Golf Greens in Scandinavia. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2013. [Google Scholar]
- Vosátka, M.; Dodd, J. The role of different arbuscular mycorrhizal fungi in the growth of Calamagrostis villosa and Deschampsia flexuosa, in experiments with simulated acid rain. Plant Soil 1998, 200, 251–263. [Google Scholar] [CrossRef]
- Smith, R.S.; Shiel, R.S.; Bardgett, R.D.; Millward, D.; Corkhill, P.; Rolph, G.; Hobbs, P.J.; Peacock, S. Soil microbial community, fertility, vegetation and diversity as targets in the restoration management of a meadow grassland. J. Appl. Ecol. 2003, 40, 51–64. [Google Scholar] [CrossRef]
- Körner, O.; Gutzmann, E.; Kledal, P. A dynamic model simulating the symbiotic effects in aquaponic systems. Acta Hortic. 2017, 1170, 309–316. [Google Scholar] [CrossRef]
- Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J.E.; Rachmilevitch, S. Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 2012, 63, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Stevanović, B.; Pavlović, P. Assessment of the Phytoremediation Potential and an Adaptive Response of Festuca Rubra L. Sown on Fly Ash Deposits: Native Grass Has a Pivotal Role in Ecorestoration Management. Ecol. Eng. 2016, 93, 250–261. [Google Scholar] [CrossRef]
- Pereira, E.; Vázquez-De-Aldana, B.; Emeterio, L.S.; Zabalgogeazcoa, I. A Survey of Culturable Fungal Endophytes from Festuca rubra subsp. pruinosa, a Grass from Marine Cliffs, Reveals a Core Microbiome. Front. Microbiol. 2019, 9, 3321. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Ridgway, K.P.; Watson, I.J.; Fitter, A.H.; Young, J.P.W. Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol. Ecol. 2003, 12, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Bever, J.D. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol. 2016, 54, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Borts, B.V.; Skoromnaya, S.F.; Tkachenko, V.I. A Frank-Read Source Model. Open J. Met. 2020, 10, 35–44. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Wilson, G.W.T. The role of mycorrhizas in plant community structure and dynamics: Lessons from grasslands. Plant Soil 2002, 244, 319–331. [Google Scholar] [CrossRef]
- Simard, S.W.; Perry, D.A.; Jones, M.; Myrold, D.; Durall, D.M.; Molina, R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, M.; Wang, C.; Wang, J.; Wang, S.; Sun, Z.; Han, S. Global change factors influence different aspects of arbuscular mycorrhizal fungal communities. Research Square 2021. [Google Scholar] [CrossRef]
- Šmilauer, P. Communities of arbuscular mycorrhizal fungi in grassland: Seasonal variability and effects of environment and host plants. Folia Geobot. 2001, 36, 243–263. [Google Scholar] [CrossRef]
- Vaida, I.; Păcurar, F.; Rotar, I.; Tomoș, L.; Stoian, V. Changes in Diversity Due to Long-Term Management in a High Natural Value Grassland. Plants 2021, 10, 739. [Google Scholar] [CrossRef]
- Vaida, I. The Influence of Management on the Agronomic and Ecological Value of Mountain Grasslands. Ph.D. Thesis, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania, 2018. Available online: https://rei.gov.ro/ (accessed on 10 November 2021).
- Rotar, I.; Carlier, L. Cultura Pajistilor; Risoprint: Cluj-Napoca, Romania, 2010; ISBN 978-973-53-0420-1. [Google Scholar]
- Stoian, V.; Vidican, R.; Rotar, I.; Păcurar, F. Mycorrhizal induced domination of Festuca rubra in grasslands. Rom. J. Grassl. Forage Crops 2016, 13, 77. [Google Scholar]
- Bothe, H.; Turnau, K.; Regvar, M. The potential role of arbuscular mycorrhizal fungi in protecting endangered plants and habitats. Mycorrhiza 2010, 20, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Fire Effects Information System (FEIS) Syntheses About Fire Ecology and Fire Regimes in the United States. Available online: https://www.fs.fed.us/database/feis/plants/graminoid/fesrub/all.html (accessed on 10 November 2021).
- Brown, R.N.; Percivalle, C.; Narkiewicz, S.; DeCuollo, S. Relative Rooting Depths of Native Grasses and Amenity Grasses with Potential for Use on Roadsides in New England. HortScience 2010, 45, 393–400. [Google Scholar] [CrossRef]
- Stoian, V.H.; Florian, V. Mycorrhiza–Benefits, Influence, Diagnostic Method. Bull. UASMV Agric. 2009, 66, 2009. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Inc.: Boston, MA, USA, 2019. [Google Scholar]
- RCore Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, R Package Version 1.9.12; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular mycorrhizas traits and yield of winter wheat profiled by mineral fertilization. Agronomy 2020, 10, 846. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Vâtcă, S.; Vidican, R.; Gâdea, Ș.; Horvat, M.; Vâtcă, A.; Stoian, V.A.; Stoian, V. Blackcurrant Variety Specific Growth and Yield Formation as a Response to Foliar Fertilizers. Agronomy 2020, 10, 2014. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2019. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 November 2021).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionaryanalyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legrende, P.; McGlinn, D.; Wagner, H. 633 Vegan: Community Ecology Package, R Package Version 2.5-2; 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 November 2021).

| Frequency (%) | Intensity (%) | Arbuscules (%) | Colonization Degree (%) | Non-Mycorrhizal Areas (%) | Mycorrhizal/Non-Mycorrhizal Area Report | |
|---|---|---|---|---|---|---|
| M1 | 6.55 ± 0.38 e | 3.59 ± 0.19 e | 0.14 ± 0.07 c | 0.42 ± 0.03 e | 96.41 ± 0.19 a | 0.04 ± 0.00 e |
| M2 | 30.82 ± 0.49 d | 13.17 ± 0.31 d | 1.54 ± 0.33 bc | 4.18 ± 0.15 d | 86.83 ± 0.31 b | 0.15 ± 0.00 d |
| M3 | 51.27 ± 0.47 c | 20.09 ± 0.51 c | 1.61 ± 0.40 bc | 10.47 ± 0.33 c | 79.91 ± 0.51 c | 0.26 ± 0.01 c |
| M4 | 71.58 ± 0.48 b | 27.65 ± 0.67 b | 2.76 ± 0.52 b | 20.02 ± 0.56 b | 72.35 ± 0.67 d | 0.41 ± 0.02 b |
| M5 | 92.69 ± 0.52 a | 38.44 ± 0.86 a | 5.21 ± 0.78 a | 35.91 ± 0.91 a | 61.55 ± 0.86 e | 0.68 ± 0.03 a |
| F test | 22748 | 3103 | 77.97 | 3038 | 3103 | 1433 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cluster | Frequency (%) | Intensity (%) | Arbuscules (%) | Colonization Degree (%) | Non-Colonized Areas (%) | Mycorrhizal/Non-Mycorrhizal Area Reports |
|---|---|---|---|---|---|---|
| C1 | 41.00 | 14.27 | 0.00 | 9.13 | 85.73 | 0.20 |
| C2 | 19.47 | 8.26 | 0.33 | 2.59 | 91.73 | 0.09 |
| C3 | 51.73 | 20.80 | 1.40 | 14.04 | 79.20 | 0.30 |
| C4 | 57.80 | 23.73 | 5.27 | 17.06 | 76.26 | 0.37 |
| C5 | 62.87 | 29.67 | 7.87 | 23.65 | 70.33 | 0.52 |
| C6 | 84.67 | 39.33 | 16.53 | 35.21 | 60.67 | 0.77 |
| C7 | 73.13 | 33.67 | 0.73 | 25.24 | 66.33 | 0.53 |
| C8 | 84.13 | 38.26 | 8.00 | 33.21 | 33.21 | 0.67 |
| C9 | 40.53 | 18.87 | 8.53 | 12.90 | 81.13 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corcoz, L.; Păcurar, F.; Pop-Moldovan, V.; Vaida, I.; Stoian, V.; Vidican, R. Mycorrhizal Patterns in the Roots of Dominant Festuca rubra in a High-Natural-Value Grassland. Plants 2022, 11, 112. https://doi.org/10.3390/plants11010112
Corcoz L, Păcurar F, Pop-Moldovan V, Vaida I, Stoian V, Vidican R. Mycorrhizal Patterns in the Roots of Dominant Festuca rubra in a High-Natural-Value Grassland. Plants. 2022; 11(1):112. https://doi.org/10.3390/plants11010112
Chicago/Turabian StyleCorcoz, Larisa, Florin Păcurar, Victoria Pop-Moldovan, Ioana Vaida, Vlad Stoian, and Roxana Vidican. 2022. "Mycorrhizal Patterns in the Roots of Dominant Festuca rubra in a High-Natural-Value Grassland" Plants 11, no. 1: 112. https://doi.org/10.3390/plants11010112
APA StyleCorcoz, L., Păcurar, F., Pop-Moldovan, V., Vaida, I., Stoian, V., & Vidican, R. (2022). Mycorrhizal Patterns in the Roots of Dominant Festuca rubra in a High-Natural-Value Grassland. Plants, 11(1), 112. https://doi.org/10.3390/plants11010112









