Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure
2.2. Photophysical Properties
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis
Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate (2)
3.3. X-Ray Diffraction Studies
3.4. Optical Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wedal, J.C.; Evans, W.J. A Rare-Earth Metal Retrospective to Stimulate All Fields. J. Am. Chem. Soc. 2021, 143, 18354–18367. [Google Scholar] [CrossRef]
- Schumann, H.; Meese-Marktscheffel, J.A.; Esser, L. Synthesis, Structure, and Reactivity of Organometallic. Pi.-Complexes of the Rare Earths in the Oxidation State Ln3+ with Aromatic Ligands. Chem. Rev. 1995, 95, 865–986. [Google Scholar] [CrossRef]
- Ortu, F. Rare Earth Starting Materials and Methodologies for Synthetic Chemistry. Chem. Rev. 2022, 122, 6040–6116. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Tutorial on the Role of Cyclopentadienyl Ligands in the Discovery of Molecular Complexes of the Rare-Earth and Actinide Metals in New Oxidation States. Organometallics 2016, 35, 3088–3100. [Google Scholar] [CrossRef]
- Behrsing, T.; Blair, V.L.; Jaroschik, F.; Deacon, G.B.; Junk, P.C. Rare Earths—The Answer to Everything. Molecules 2024, 29, 688. [Google Scholar] [CrossRef]
- Deacon, G.B.; Jaroschik, F.; Junk, P.C.; Kelly, R.P. Bulky Group 2 Octaphenylmetallocenes and Direct Access to Calcium and Ytterbium Pseudo-Grignard Complexes. Organometallics 2015, 34, 2369–2377. [Google Scholar] [CrossRef]
- Harder, S.; Ruspic, C. Insight in Cyclopentadienyl Metal Complexes with Superbulky Ligands: The Crystal Structure of [CpBIGK]∞. J. Organomet. Chem. 2009, 694, 1180–1184. [Google Scholar] [CrossRef]
- Harder, S.; Naglav, D.; Ruspic, C.; Wickleder, C.; Adlung, M.; Hermes, W.; Eul, M.; Pöttgen, R.; Rego, D.B.; Poineau, F.; et al. Physical Properties of Superbulky Lanthanide Metallocenes: Synthesis and Extraordinary Luminescence of [EuII(CpBIG)2] (CpBIG=(4-NBu-C6H4)5-Cyclopentadienyl). Chem.–A Eur. J. 2013, 19, 12272–12280. [Google Scholar] [CrossRef]
- Evans, W.J. The Organometallic Chemistry of the Lanthanide Elements in Low Oxidation States. Polyhedron 1987, 6, 803–835. [Google Scholar] [CrossRef]
- Roitershtein, D.M.; Puntus, L.N.; Vinogradov, A.A.; Lyssenko, K.A.; Minyaev, M.E.; Dobrokhodov, M.D.; Taidakov, I.V.; Varaksina, E.A.; Churakov, A.V.; Nifant’Ev, I.E. Polyphenylcyclopentadienyl Ligands as an Effective Light-Harvesting μ-Bonded Antenna for Lanthanide +3 Ions. Inorg. Chem. 2018, 57, 10199–10213. [Google Scholar] [CrossRef] [PubMed]
- Puntus, L.N.; Varaksina, E.A.; Bardonov, D.A.; Minyaev, M.E.; Lyssenko, K.A.; Chernenkiy, T.A.; Taydakov, I.V.; Nifant’ev, I.E.; Roitershtein, D.M. Efficiency of the Energy Transfer through 5d State of the Ln3+ Ion in Complexes with Diarylcyclopentadienyl Ligands. Opt. Mater. 2025, 160, 116734. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Sloop, J.C.; Bumgardner, C.L.; Washington, G.; Loehle, W.D.; Sankar, S.S.; Lewis, A.B. Keto—Enol and Enol—Enol Tautomerism in Trifluoromethyl-b-Diketones. J. Fluor. Chem. 2006, 127, 780–786. [Google Scholar] [CrossRef]
- Komarov, P.D.; Birin, K.P.; Vinogradov, A.A.; Varaksina, E.A.; Puntus, L.N.; Lyssenko, K.A.; Churakov, A.V.; Nifant’ev, I.E.; Minyaev, M.E.; Roitershtein, D.M. Coordination Polymers of Polyphenyl-Substituted Potassium Cyclopentadienides. Molecules 2022, 27, 7725. [Google Scholar] [CrossRef]
- Sato, S.; Masanobu, W. Relations between Intramolecular Energy Transfer Efficiencies and Triplet State Energies in Rare Earth β-Diketone Chelates. Bull. Chem. Soc. Jpn. 1970, 43, 1955–1962. [Google Scholar] [CrossRef]
- Bardonov, D.A.; Puntus, L.N.; Taidakov, I.V.; Varaksina, E.A.; Lyssenko, K.A.; Nifant’ev, I.E.; Roitershtein, D.M. Ligand-to-Ligand Charge Transfer State in Lanthanide Complexes Containing p-Bonded Antenna Ligands. Mendeleev Commun. 2022, 32, 198–201. [Google Scholar] [CrossRef]
- Puntus, L.; Bünzli, J.-C.G. Influence of Charge Transfer States on Lanthanide Photophysics. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthan; Wolfbeis, O.S., Ed.; Lumin: Christchurch, New Zealand; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–46. [Google Scholar]
- Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Font-Bardía, M.; Tubau, À.; Speed, S.; Massoud, S.S. Diverse Coordination Numbers and Geometries in Pyridyl Adducts of Lanthanide(III) Complexes Based on β-Diketonate. Inorganics 2021, 9, 74. [Google Scholar] [CrossRef]
- Aléo, A.D.; Pointillart, F.; Ouahab, L.; Andraud, C.; Maury, O. Charge Transfer Excited States Sensitization of Lanthanide Emitting from the Visible to the Near-Infra-Red. Coord. Chem. Rev. 2012, 256, 1604–1620. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Y.; Xue, N.; Qu, J. Synthesis of Trifluoromethyl Ketones via Tandem Claisen Condensation and Retro-Claisen C–C Bond-Cleavage Reaction. J. Org. Chem. 2013, 78, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.F.; Woen, D.H.; Mohanam, L.N.; Ziller, J.W.; Furche, F.; Evans, W.J. Tetramethylcyclopentadienyl Ligands Allow Isolation of Ln(II) Ions across the Lanthanide Series in [K(2.2.2-Cryptand)][(C5Me4H)3Ln] Complexes. Organometallics 2018, 37, 3863–3873. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.42; Rigaku Oxford Diffraction: Wroclaw, Poland, 2023.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
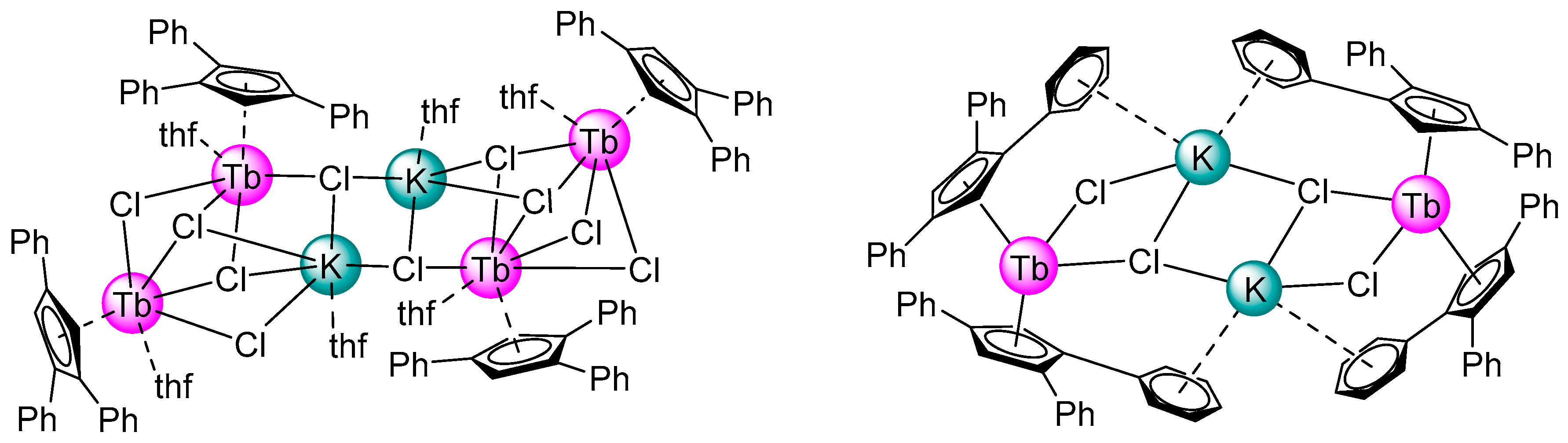
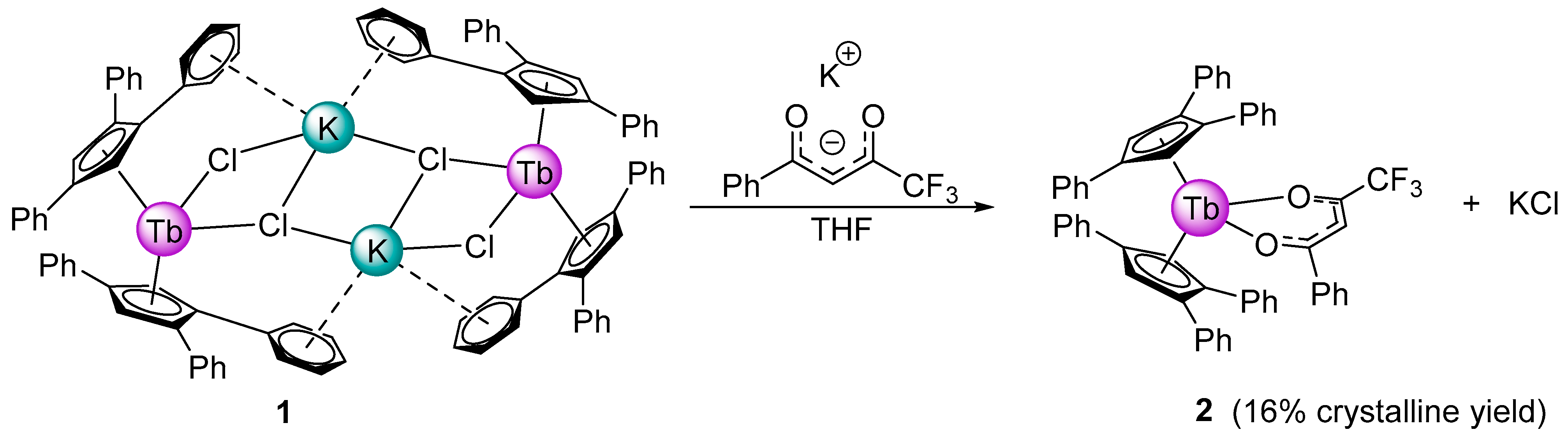
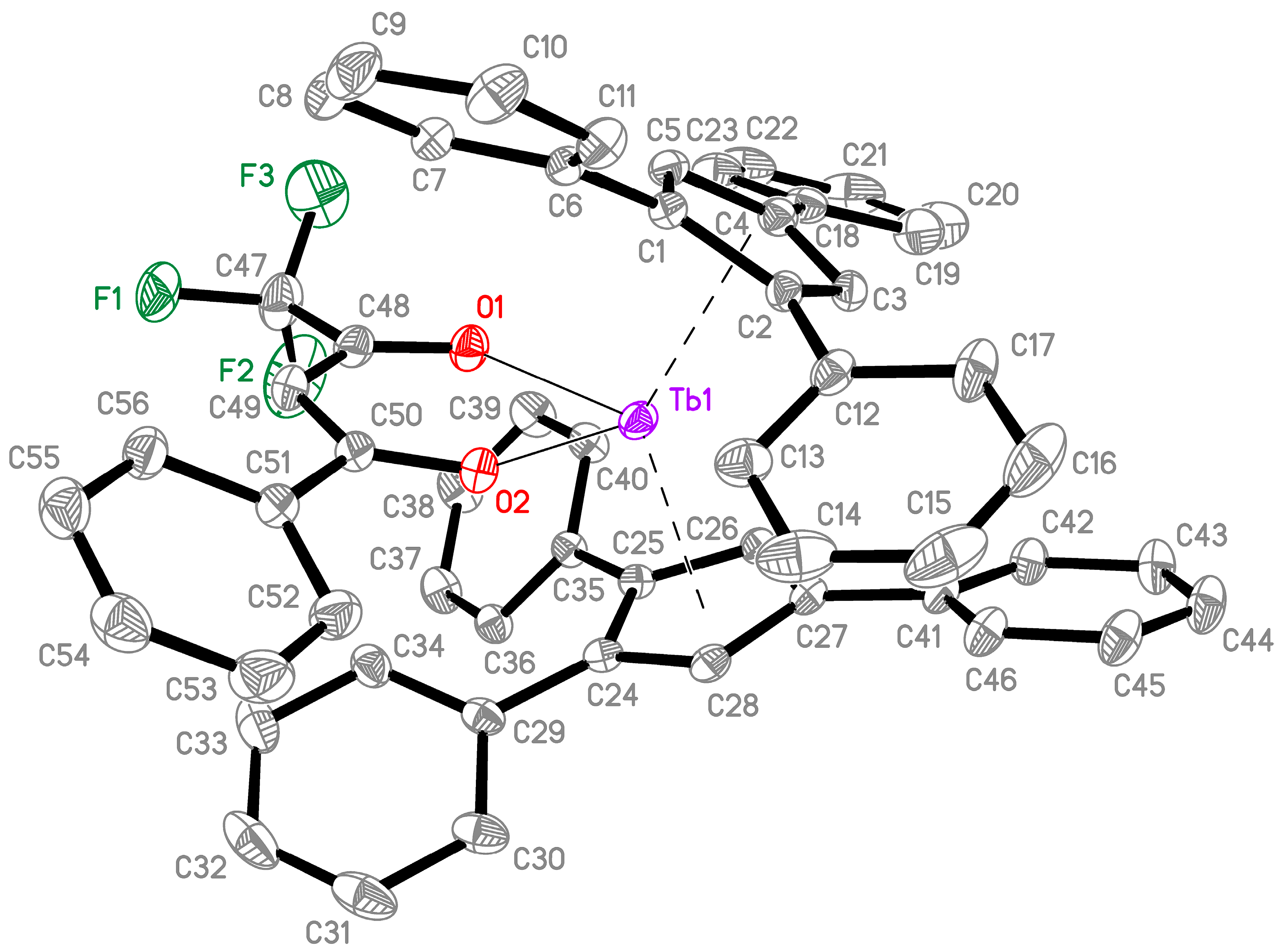
| Disnances and Angles | 2 | 1 [10] |
|---|---|---|
| Tb-Cpcent (Å) | 2.385, 2.400 | 2.422, 2.429 |
| Cpcent-Tb-Cpcent (◦) | 133.9 | 132.0 |
| Tb-CCp(range) (Å) | 2.645(1)–2.721(1) | 2.656(3)–2.751(3) |
| Tb-CCp(average) (Å) | 2.680(1) | 2.691(3) |
| Tb-O (Å) | 2.2126(9), 2.2428(8) | - |
| O-Tb-O, (Cl–TbCl) (◦) | 76.64(3) | 91.72(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardonov, D.A.; Minyaev, M.E.; Puntus, L.N.; Nifant’ev, I.E.; Roitershtein, D.M. Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate. Molbank 2025, 2025, M2102. https://doi.org/10.3390/M2102
Bardonov DA, Minyaev ME, Puntus LN, Nifant’ev IE, Roitershtein DM. Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate. Molbank. 2025; 2025(4):M2102. https://doi.org/10.3390/M2102
Chicago/Turabian StyleBardonov, Daniil A., Mikhail E. Minyaev, Lada N. Puntus, Ilya E. Nifant’ev, and Dmitrii M. Roitershtein. 2025. "Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate" Molbank 2025, no. 4: M2102. https://doi.org/10.3390/M2102
APA StyleBardonov, D. A., Minyaev, M. E., Puntus, L. N., Nifant’ev, I. E., & Roitershtein, D. M. (2025). Bis(1,2,4-triphenylcyclopentadienyl) Terbium 4,4,4-trifluoro-1-phenylbutane-1,3-dionate. Molbank, 2025(4), M2102. https://doi.org/10.3390/M2102







