4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one
Abstract
1. Introduction
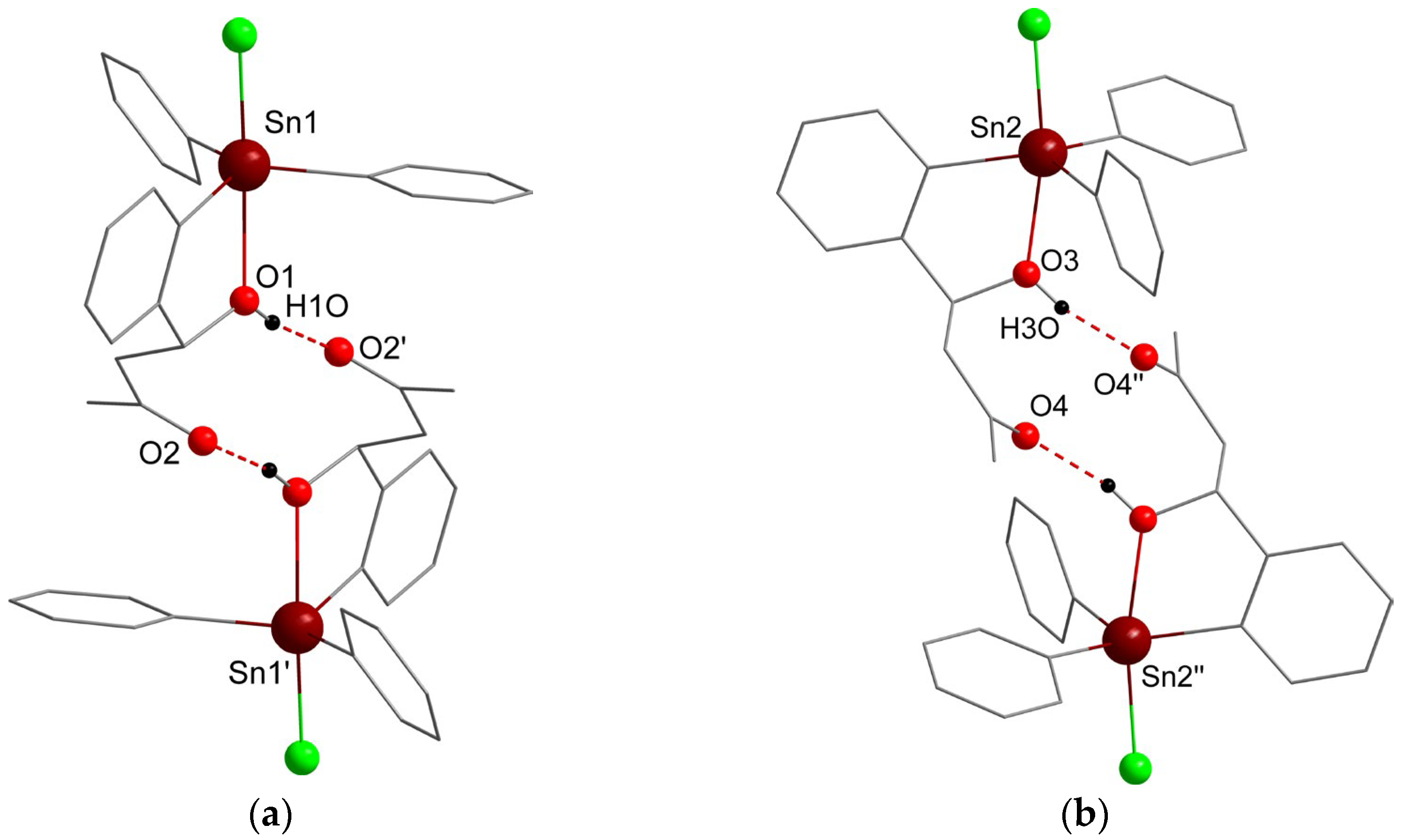
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one 1
3.2. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kašná, B.; Jambor, R.; Dostál, L.; Růžička, A.; Císařová, I.; Holeček, J. Quest for Organotin(IV) Cations Containing O,C,O-Chelating Ligands. Organometallics 2004, 23, 5300–5307. [Google Scholar] [CrossRef]
- Kašná, B.; Jambor, R.; Dostál, L.; Císařová, I.; Holeček, J. Double O,C,O-chelated diorganotin(IV) derivatives. J. Organomet. Chem. 2006, 691, 1554–1559. [Google Scholar] [CrossRef]
- Dostál, L.; Jambor, R.; Růžička, A.; Císařová, I.; Holeček, J.; Biesemans, M.; Willem, R.; De Proft, F.; Geerlings, P. Organotin(IV) Derivatives of Some O,C,O-Chelating Ligands. Part 2. Organometallics 2007, 26, 6312–6319. [Google Scholar] [CrossRef]
- Novak, M.; Dostal, L.; Ruzicka, A.; Jambor, R. Organotin(IV) compounds containing N,C,O-chelating ligand. Inorg. Chim. Acta 2014, 410, 20–28. [Google Scholar] [CrossRef]
- Hébert, M.; Petiot, P.; Benoit, E.; Dansereau, J.; Ahmad, T.; Le Roch, A.; Ottenwaelder, X.; Gagnon, A. Synthesis of Highly Functionalized Triarylbismuthines by Functional Group Manipulation and Use in Palladium- and Copper-Catalyzed Arylation Reactions. J. Org. Chem. 2016, 81, 5401–5416. [Google Scholar] [CrossRef]
- Deak, N.; Thillaye du Boullay, O.; Moraru, I.-T.; Mallet-Ladeira, S.; Madec, D.; Nemes, G. A non-symmetric sulfur-based O,C,O-chelating pincer ligand leading to chiral germylene and stannylene. Dalton Trans. 2019, 48, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pau, J.; Loungxay, J.; Magobenny, T.; Wylie, R.S.; Lough, A.J.; Foucher, D. Hypercoordinated organotin(IV) compounds containing C,O- and C,N- chelating ligands: Synthesis, characterisation, DFT studies and polymerization behaviour. J. Organomet. Chem. 2019, 900, 120910. [Google Scholar] [CrossRef]
- Barbul, I.; Varga, R.A.; Silvestru, C. Structural diversity of coordination cores in homoleptic tetraaryltin(IV) dioxolane, aldehyde and imines: The first octacoordinated double helicate tetraorganotin(IV) compound. Eur. J. Inorg. Chem. 2013, 2013, 3146–3154. [Google Scholar] [CrossRef]
- Sharma, P.; Pérez, D.; Rosas, N.; Cabrera, A.; Toscano, A. New stibines containing acetal and formyl group: Platinum complex and unexpected oxastibol derivative. J. Organomet. Chem. 2006, 691, 579–584. [Google Scholar] [CrossRef]
- Pöllnitz, A.; Silvestru, C.; Carpentier, J.-F.; Silvestru, A. Diorganodiselenides and zinc(II) organoselenolates containing (imino)aryl groups of type 2-(RN=CH)C6H4. Dalton Trans. 2012, 41, 5060–5070. [Google Scholar] [CrossRef]
- Someșan, A.-A.; Vieriu, S.-M.; Crăciun, A.; Silvestru, C.; Chiroi, P.; Nutu, A.; Jurj, A.; Lajos, R.; Berindan-Neagoe, I.; Varga, R.A. C,O-Chelated organotin(IV) derivatives as potential anticancer agents: Synthesis, characterization, and cytotoxic activity. Appl. Organomet. Chem. 2022, 36, e6540. [Google Scholar] [CrossRef]
- Murafuji, T.; Mutoh, T.; Satoh, K.; Tsunenari, K.; Azuma, N.; Suzuki, H. Hypervalent Bond Formation in Halogeno(2-acylphenyl)bismuthanes. Organometallics 1995, 14, 3848–3854. [Google Scholar] [CrossRef]
- Obata, T.; Matsumura, M.; Kawahata, M.; Hoshino, S.; Yamada, M.; Murata, Y.; Kakusawa, N.; Yamaguchi, K.; Tanaka, M.; Yasuike, S. Synthesis, structural characterization and antitumor activity of 2-(di-p-tolylstibano)- and 2-(di-p-tolylbismuthano)-N-p-tolylbenzamide. J. Organomet. Chem. 2016, 807, 17–21. [Google Scholar] [CrossRef]
- Barbul, I.; Varga, R.A.; Molloy, K.C.; Silvestru, C. Di(imino)aryltin(IV) dichlorides as tectons for heterometallic coordination compounds. Dalton Trans. 2013, 42, 15427–15436. [Google Scholar] [CrossRef] [PubMed]
- Somesan, A.-A.; Barbul, I.; Vieriu, S.-M.; Varga, R.A.; Silvestru, C. Novel mono- and bimetallic organotin(IV) compounds as potential linkers for coordination polymers. Dalton Trans. 2019, 48, 6527–6538. [Google Scholar] [CrossRef]
- Someşan, A.-A.; Silvestru, C.; Varga, R.A. Reactivity of a carbonyl moiety in organotin(IV) compounds: Novel Pd(II) and Cu(II) complexes supported by organotin(iv) ligands. New J. Chem. 2021, 45, 3817–3827. [Google Scholar] [CrossRef]
- Afloarei, C.; Barbul, I.; Someșan, A.-A.; Silvestru, C.; Varga, R.A. Supramolecular architectures in novel diphenyl(aryl)tin(IV) chlorides. Polyhedron 2022, 222, 115894. [Google Scholar] [CrossRef]
- Smith, M.B. March’s Organic Chemistry: Reactions, Mechanisms, and Structure, 7th ed.; John Wiley: New York, NY, USA, 2013. [Google Scholar]
- Perrin, C.L.; Chang, K.-L. The Complete Mechanism of an Aldol Condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Qian, C.; An, L.; Wang, W.; Li, X.; Shao, X.; Li, Z. Research progress of catalysts for aldol condensation of biomass based compounds. RSC Adv. 2023, 13, 9466–9478. [Google Scholar] [CrossRef]
- Bohre, A.; Alam, M.I.; Avasthi, K.; Ruiz-Zepeda, F.; Likozar, B. Low temperature transformation of lignocellulose derived bioinspired molecules to aviation fuel precursor over magnesium–lanthanum mixed oxide catalyst. Appl. Catal. B Environ. 2020, 276, 119069. [Google Scholar] [CrossRef]
- Mukaiyama, T. The Directed Aldol Reaction. Org. React. 2005, 28, 203–331. [Google Scholar]
- Song, X.; Liu, A.-X.; Liu, S.-S.; Gao, W.-C.; Wang, M.-C.; Chang, J. Enantiopure azetidine-2-carboxamides as organocatalysts for direct asymmetric aldol reactions in aqueous and organic media. Tetrahedron 2014, 70, 1464–1470. [Google Scholar] [CrossRef]
- Shvartsbart, A.; Smith, A.B., III. The Daphniphyllum Alkaloids: Total Synthesis of (−)-Calyciphylline N. J. Am. Chem. Soc. 2015, 137, 3510–3519. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-H.; Hwang, J.; Han, J.W.; Hwang, K.-R. Carbon recovery from wasted aqueous-phase bio-oil to fuel precursors through aldol-condensation reaction: A comprehensive review. J. Ind. Eng. Chem. 2023, 126, 115–126. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, J.; Chen, J.; Liu, R.; Qin, Z.; Chen, H.; Yue, W. Aldol Condensation for the Construction of Organic Functional Materials. Angew. Chem. Int. Ed. 2024, 63, e202311879. [Google Scholar] [CrossRef]
- Holeček, J.; Handliř, K.; Nádvorník, M.; Lyčka, A. Dependence of [1J(119Sn,13C)] on the mean C–Sn–C Angle in Phenyltin(IV) Compounds. Z. Chem. 1990, 30, 265–266. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds: Tables of Spectral Data. In Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2000; p. 287. [Google Scholar]
- Mestrelab Research, S.L. MestReC and MestReNova, version 12.0.4; Mestrelab Research S.L.: Santiago de Compostela, Spain, 2015.
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Crystal Impact. DIAMOND–Visual Crystal Structure Information System, version 5.0.0 build 45; Crystal Impact: Bonn, Germany, 2023.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Someșan, A.-A.; Varga, R.A. 4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one. Molbank 2025, 2025, M1991. https://doi.org/10.3390/M1991
Someșan A-A, Varga RA. 4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one. Molbank. 2025; 2025(2):M1991. https://doi.org/10.3390/M1991
Chicago/Turabian StyleSomeșan, Adrian-Alexandru, and Richard A. Varga. 2025. "4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one" Molbank 2025, no. 2: M1991. https://doi.org/10.3390/M1991
APA StyleSomeșan, A.-A., & Varga, R. A. (2025). 4-[2-(Chlorodiphenylstannyl)phenyl]-4-hydroxybutan-2-one. Molbank, 2025(2), M1991. https://doi.org/10.3390/M1991






