Synthesis and Structural Characterization of Cobalt Complexes Ligated by N-Methyl(bis(diphenylphosphino)amine)
Abstract
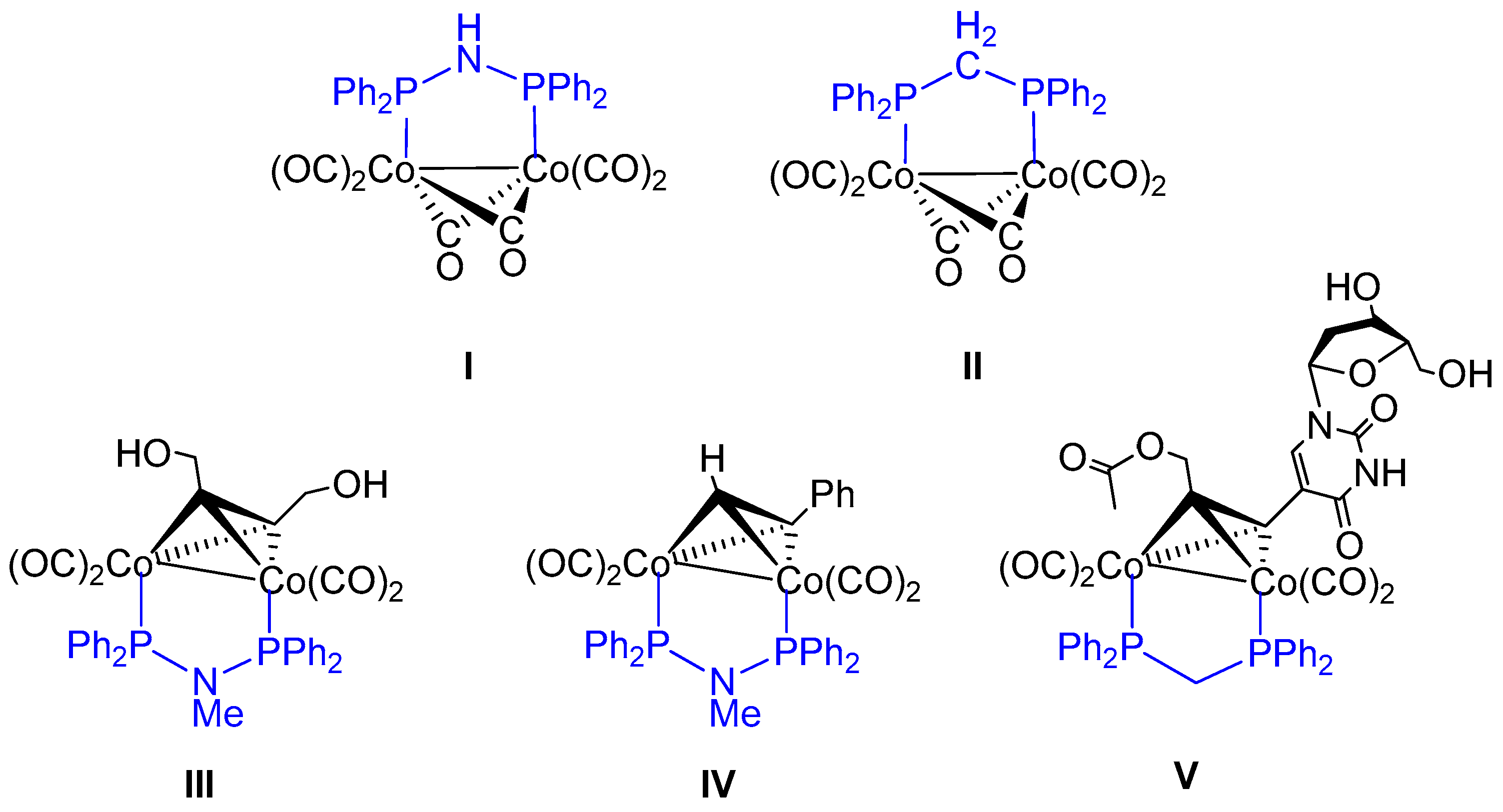
1. Introduction
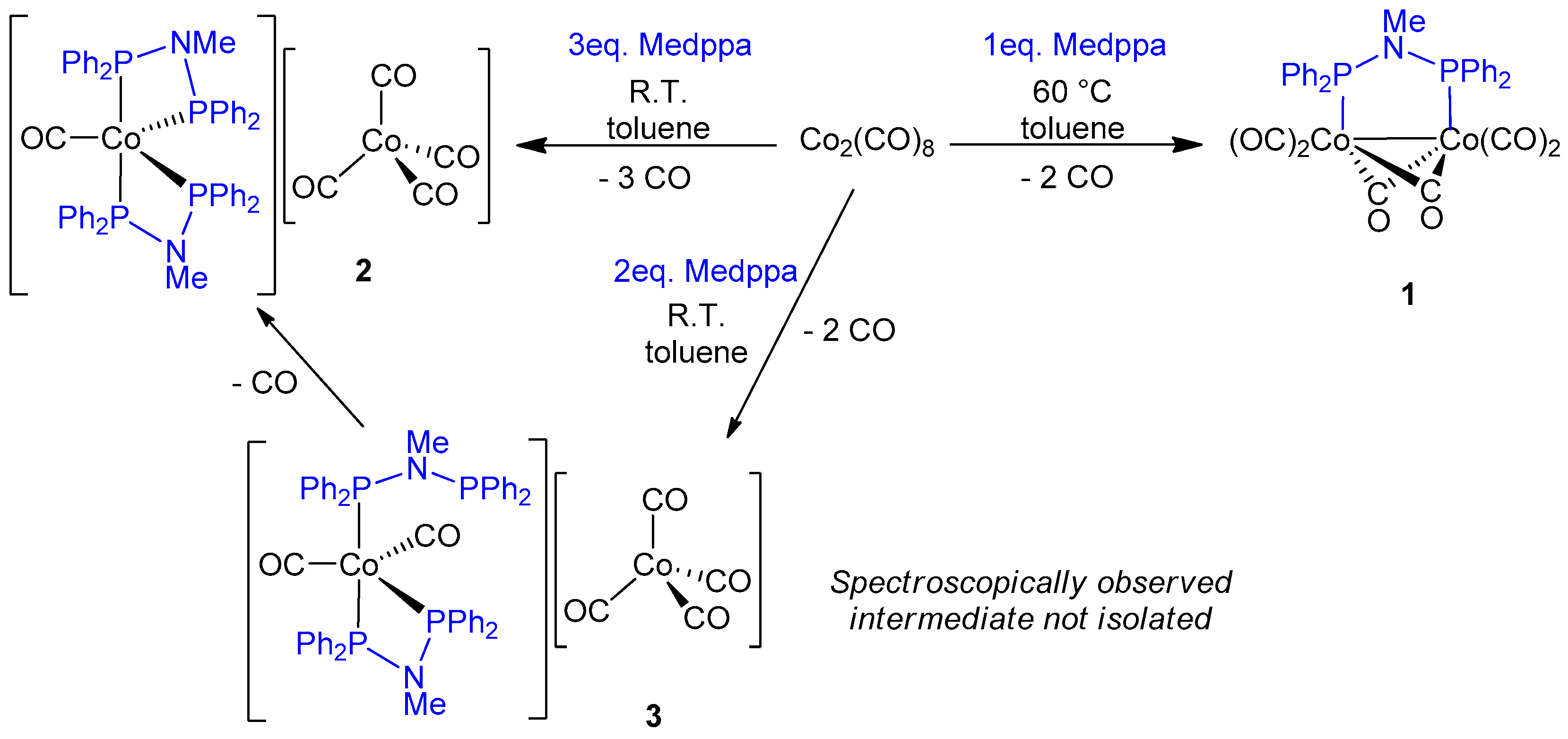
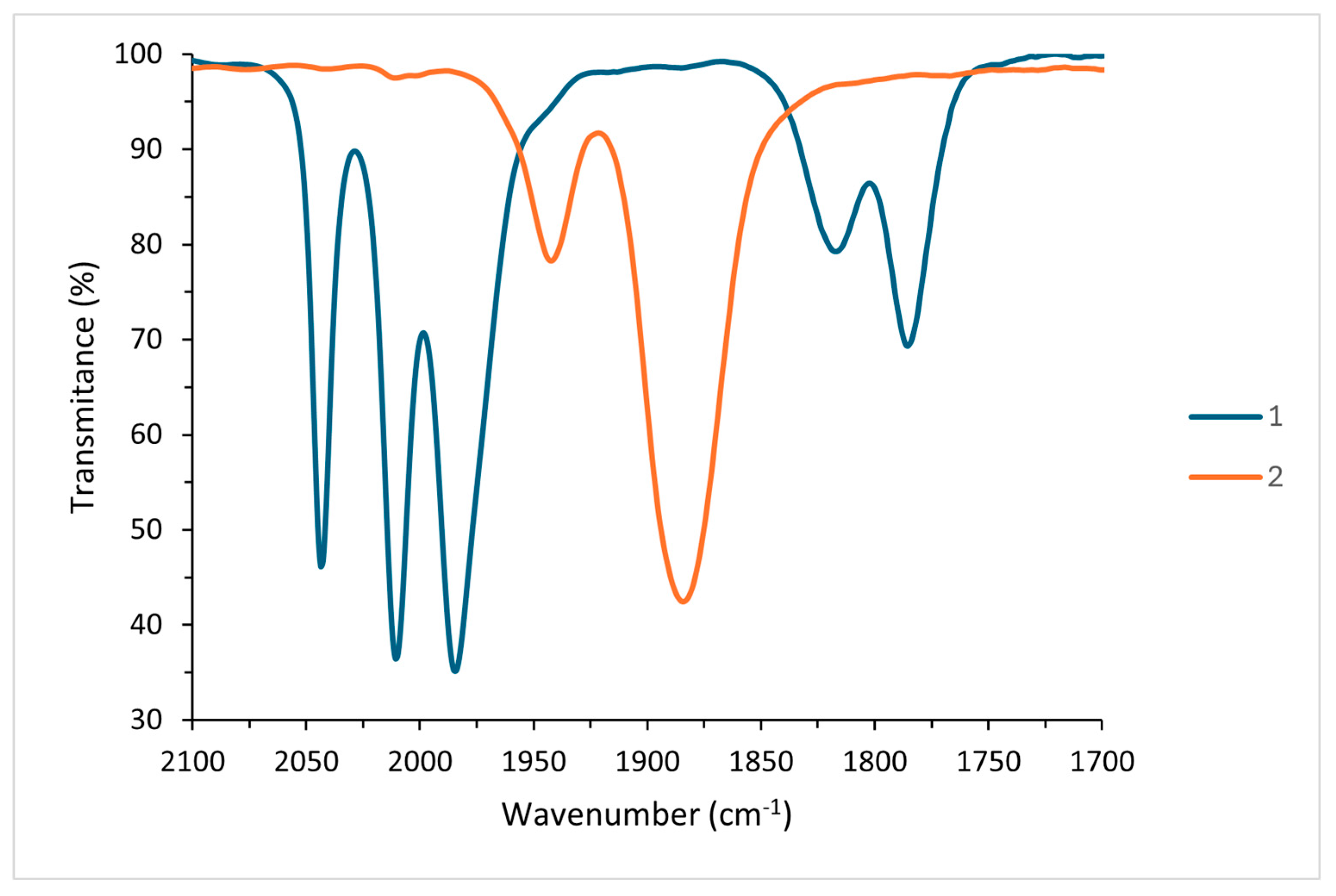
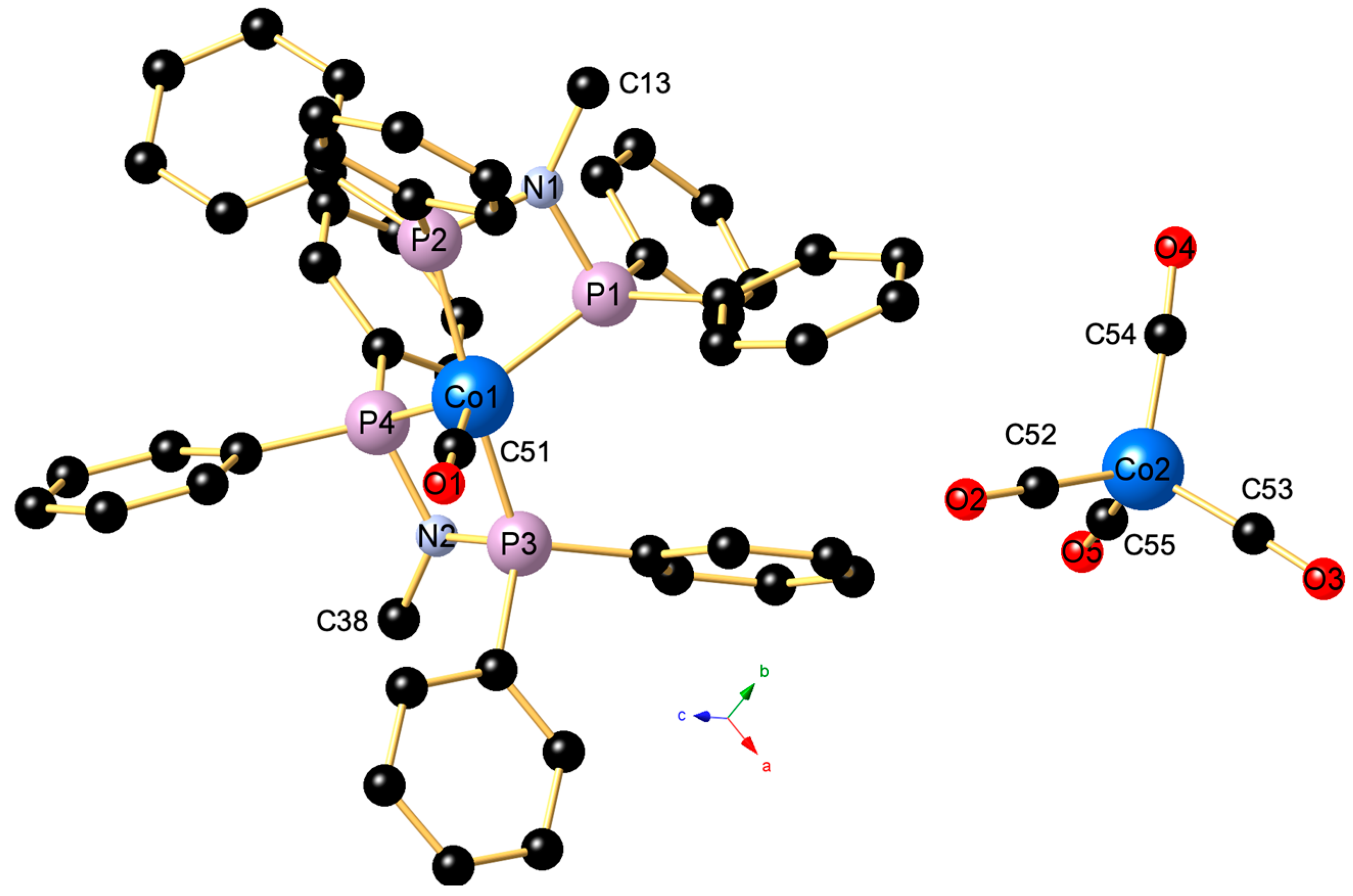
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manning, A.R. Infrared Spectra of Some Derivatives of Octacarbonyldicobalt. J. Chem. Soc. Inorg. Phys. Theor. 1968, 1135–1137. [Google Scholar] [CrossRef]
- Booth, B.L.; Gardner, M.; Haszeldine, R.N. A Carbonyl-Bridged, Phosphite-Substituted Cobalt Carbonyl Derivative. J. Chem. Soc. Chem. Commun. 1969, 1388–1389. [Google Scholar] [CrossRef]
- Haumann, M.; Meijboom, R.; Moss, J.R.; Roodt, A. Synthesis, Crystal Structure and Hydroformylation Activity of Triphenylphosphite Modified Cobalt Catalysts. Dalton Trans. 2004, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, I.; Knorr, M.; Brieger, L.; Strohmann, C. Synthesis of Tris(Arylphosphite)-Ligated Cobalt(0) Complexes [Co2(CO)6{P(OAr)3}2], and Their Reactivity towards Phenylacetylene and Diphenylacetylene. Adv. Chem. Res. 2020, 2, 1–20. [Google Scholar] [CrossRef]
- Casati, N.; Macchi, P.; Sironi, A. Molecular Crystals under High Pressure: Theoretical and Experimental Investigations of the Solid-Solid Phase Transitions in [Co2(CO)6(XPh3)2] (X = P, As). Chem. Eur. J. 2009, 15, 4446–4457. [Google Scholar] [CrossRef]
- Bartik, T.; Bartik, B.; Hanson, B.E.; Whitmire, K.H.; Guo, I. Reactions of Trisulfonated Triphenylphosphine, TPPTS, with Cobalt Carbonyls in Water. Inorg. Chem. 1993, 32, 5833–5837. [Google Scholar] [CrossRef]
- Weber, R.; Englert, U.; Ganter, B.; Keim, W.; Möthrath, M. Hydroformylation of Epoxides Catalyzed by Cobalt and Hemilabile P–O Ligands. Chem. Commun. 2000, 1419–1420. [Google Scholar] [CrossRef]
- Meijboom, R.; Haumann, M.; Roodt, A.; Damoense, L. Synthesis, Spectroscopy, and Hydroformylation Activity of Sterically Demanding, Phosphite-Modified Cobalt Catalysts. Helv. Chim. Acta 2005, 88, 676–693. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, P.; Lai, E.; Li, J.; Chen, Y.; Jiang, W.; Wang, B.; Zhu, H. Cobalt Carbonyls Stabilized by N,P-Ligands: Synthesis, Structure, and Catalytic Property for Ethylene Oxide Hydroalkoxycarbonylation. Chem. Asian J. 2021, 16, 3453–3461. [Google Scholar] [CrossRef]
- Amézquita-Valencia, M.; Ramírez-Garavito, R.; Toscano, R.A.; Cabrera, A. Hydrogenation of α-Enaminoketones with Cobalt Phosphine-Modified Catalysts. Catal. Commun. 2013, 33, 29–33. [Google Scholar] [CrossRef]
- Pauson, P.L.; Khand, I.U. Uses of Cobalt-Carbonyl Acetylene Complexes in Organic Synthesis. Ann. N. Y. Acad. Sci. 1977, 295, 2–14. [Google Scholar] [CrossRef]
- Gibson, S.E.; Stevenazzi, A. The Pauson–Khand Reaction: The Catalytic Age Is Here! Angew. Chem. Int. Ed. 2003, 42, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Urgoiti, J.; Añorbe, L.; Pérez-Serrano, L.; Domínguez, G.; Pérez-Castells, J. The Pauson–Khand Reaction, a Powerful Synthetic Tool for the Synthesis of Complex Molecules. Chem. Soc. Rev. 2004, 33, 32–42. [Google Scholar] [CrossRef]
- Golovko, V.B.; Hope-Weeks, L.J.; Mays, M.J.; McPartlin, M.; Sloan, A.M.; Woods, A.D. Synthesis of Cobalt-Containing Cyclophanes, and the Formation of an Unprecedented Seven-Membered Cyclic Diyne. New J. Chem. 2004, 28, 527–534. [Google Scholar] [CrossRef]
- Mague, J.T. “Short-Bite” Ligands in Cluster Synthesis. J. Clust. Sci. 1995, 6, 217–269. [Google Scholar] [CrossRef]
- Anandhi, U.; Holbert, T.; Lueng, D.; Sharp, P.R. Platinum and Palladium Imido and Oxo Complexes with Small Natural Bite Angle Diphosphine Ligands. Inorg. Chem. 2003, 42, 1282–1295. [Google Scholar] [CrossRef]
- Ellermann, J.; Geheeb, N.; Zoubek, G.; Thiele, G. Complex Chemistry of Polyfunctional Ligands, 411 Synthesis and Crystal Structure of Di-µ-Carbonyl-µ-Bis(Diphenylphosphino)Amine-Tetracarbonyl-Dicobalt(0) (Co—Co) · 0.5 Benzene. Z. Für Naturforsch. B 1977, 32, 1271–1276. [Google Scholar] [CrossRef]
- Chang; Lee, J.-C.; Hong, F.-E. Design, Synthesis, Application, and DFT Investigation of Suzuki−Miyaura Reactions of a Dicobalt Carbonyl-Containing Phosphine Ligand. Organometallics 2005, 24, 5686–5695. [Google Scholar] [CrossRef]
- Lisic, E.C.; Hanson, B.E. Solution Structure and Dynamics of Co2(CO)6(L-L) and Co4(CO)8(L-L)2 Molecules. Inorg. Chem. 1986, 25, 812–815. [Google Scholar] [CrossRef]
- Lisic, E.C.; Hanson, B.E. Synthesis and Reactivity of Cobalt Dimers of the Type Co2(CO)3(L-L)2I2. Crystal Structure of [Co2(CO)2(µ-CO)(µ-I)(Me2PCH2PMe2)(Ph2PCH2PPh2)]I. Organometallics 1987, 6, 512–516. [Google Scholar] [CrossRef]
- Pohl, D.; Ellermann, J.; Knoch, F.A.; Moll, M.; Bauer, W. Chemie Polyfunktioneller Moleküle: CXIII Komplexe des Dicobaltoctacarbonyls mit den Liganden Bis(diphenylphosphino)amin und -amid. J. Organomet. Chem. 1994, 481, 259–274. [Google Scholar] [CrossRef]
- Hong, F.-E.; Ho, Y.-J.; Chang, Y.-C.; Lai, Y.-C. Palladium Catalyzed Suzuki Coupling Reactions Using Cobalt-Containing Bulky Phosphine Ligands. Tetrahedron 2004, 60, 2639–2645. [Google Scholar] [CrossRef]
- Huang, P.-C.; Hong, F.-E. Amination and Suzuki Coupling Reactions Catalyzed by Palladium Complexes Coordinated by Cobalt-Containing Monodentate Phosphine Ligands with Bis-Trifluoromethyl Substituents on Bridged Arylethynyl: Observation of Some Unusual Metal-Containing Compounds. J. Organomet. Chem. 2009, 694, 113–121. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Jourdain, I.; Knorr, M.; Boullanger, S.; Brieger, L.; Strohmann, C. Heterodinuclear Diphosphane-Bridged Iron–Platinum Diyne Complexes as Metalloligands for the Assembly of Polymetallic Systems (Fe, Pt, Co). J. Clust. Sci. 2019, 30, 1211–1225. [Google Scholar] [CrossRef]
- Schmidt, K.; Jung, M.; Keilitz, R.; Schnurr, B.; Gust, R. Acetylenehexacarbonyldicobalt Complexes, a Novel Class of Antitumor Drugs. Inorg. Chim. Acta 2000, 306, 6–16. [Google Scholar] [CrossRef]
- Vessières, A.; Top, S.; Vaillant, C.; Osella, D.; Mornon, J.-P.; Jaouen, G. Estradiols Modified by Metal Carbonyl Clusters as Suicide Substrates for the Study of Receptor Proteins: Application to the Estradiol Receptor. Angew. Chem. Int. Ed. Engl. 1992, 31, 753–755. [Google Scholar] [CrossRef]
- Neukamm, M.A.; Pinto, A.; Metzler-Nolte, N. Synthesis and Cytotoxicity of a Cobaltcarbonyl–Alkyne Enkephalin Bioconjugate. Chem. Commun. 2008, 232–234. [Google Scholar] [CrossRef]
- Kaczmarek, R.; Korczyński, D.; Królewska-Golińska, K.; Wheeler, K.A.; Chavez, F.A.; Mikus, A.; Dembinski, R. Organometallic Nucleosides: Synthesis and Biological Evaluation of Substituted Dicobalt Hexacarbonyl 2′-Deoxy-5-Oxopropynyluridines. ChemistryOpen 2018, 7, 237–247. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Jourdain, I.; Knorr, M.; Beffy, S.; Elmi Fourreh, A.; Siddique, F.; Chtita, S.; Strohmann, C.; Schmidt, A.; Hussien, P.M. Synthesis and Crystallographic Investigation of Dicobalt Tetrahedrane Complexes Ligated by 2-Butyne-1,4-Diol. In Silico Evaluation of Their Efficiency as Anticancer Metallodrugs. J. Mol. Struct. 2024, 1321, 140108. [Google Scholar] [CrossRef]
- Gimbert, Y.; Robert, F.; Durif, A.; Averbuch, M.-T.; Kann, N.; Greene, A.E. Synthesis and Characterization of New Binuclear Co(0) Complexes with Diphosphinoamine Ligands. A Potential Approach for Asymmetric Pauson−Khand Reactions. J. Org. Chem. 1999, 64, 3492–3497. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kochi, J.K. Oxidation-Reduction of Carbonylcobalt Cation-Anion Pairs in Coupling to Dimeric Cobalt Carbonyls. Inorg. Chem. 1989, 28, 567–578. [Google Scholar] [CrossRef]
- de Leeuw, G.; Field, J.S.; Haines, R.J.; Minshall, E.M. Neutral and Ionic Products of the Reactions of Tetra-Alkoxy and Tetraphenoxy Diphosphazane Ligands with Dicobalt Octacarbonyl: Crystal Structure of [Co(CO){(PriO)2PN(Et)P(OPri)2}2][BPh4]. S. Afr. J. Chem. 1988, 41, 9–16. [Google Scholar]
- Zhang, Z.-Z.; Yu, A.; Xi, H.-P.; Wang, R.-J.; Wang, H.-G. Formation and Structure of [Co{Ph2PN(iBu)PPh2-P,P′}2(CO)][Co)(CO)4]—A Cage Molecule-Pair or an Ion-Pair Complex? J. Organomet. Chem. 1994, 470, 223–229. [Google Scholar] [CrossRef]
- Dura, L.; Spannenberg, A.; Beweries, T. Crystal Structure of Tricarbonyl(N-diphenyl phosphanyl-N,N′-diisopropyl-p-phenyl phosphonous diamide-κP,P′)Cobalt(I) Tetracarbonyl cobaltate(−I) Toluene 0.25-Solvate. Acta Crystallogr. Sect. E 2014, 70, 533–535. [Google Scholar] [CrossRef]
- Bauer, W.; Ellermann, J.; Dotzler, M.; Pohl, D.; Heinemann, F.W.; Moll, M. Kristallstrukturelle, Festkörper-31P-CP/MAS-NMR, TOSS, 31P-COSY-NMR und mechanistische Beiträge zur Koordinationschemie des Octacarbonyldicobalts mit den Liganden Bis(diphenylphosphanyl)amin, Bis(diphenylphosphanyl)methan und 1,1,1-Tris(diphenylphosphanyl)ethan. Z. Anorg. Allg. Chem. 2000, 626, 574–587. [Google Scholar] [CrossRef]
- Cook, A.W.; Emge, T.J.; Waldie, K.M. Insights into Formate Oxidation by a Series of Cobalt Piano-Stool Complexes Supported by Bis(Phosphino)Amine Ligands. Inorg. Chem. 2021, 60, 7372–7380. [Google Scholar] [CrossRef]
- Elliot, D.J.; Holah, D.G.; Hughes, A.N.; Magnuson, V.R.; Moser, I.M.; Puddephatt, R.J.; Xu, W. New Routes to Cobalt(I) Bis(Diphenylphosphino)Methane Carbonyl Complexes Containing Monocoordinated and Chelating Ligands: Structure of [Co(η2-dppm)2(CO)][Co(CO)4]. Organometallics 1991, 10, 3933–3939. [Google Scholar] [CrossRef]
- Clément, S.; Guyard, L.; Khatyr, A.; Knorr, M.; Rousselin, Y.; Kubicki, M.M.; Mugnier, Y.; Richeter, S.; Gerbier, P.; Strohmann, C. Synthesis, Crystallographic and Electrochemical Study of Ethynyl [2.2]Paracyclophane-Derived Cobalt Metallatetrahedranes. J. Organomet. Chem. 2012, 699, 56–66. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Reber, G.; Schier, A.; Wagner, F.E.; Müiller, G. Structural Correlations between trans- and cis-Bis(diphenylphosphino)Ethene, Bis(diphenylphosphino)methane and their Chlorogold(I) Complexes. Inorg. Chim. Acta 1988, 147, 143–150. [Google Scholar] [CrossRef]
- Nöth, H.; Fluck, E. Röntgenstrukturuntersuchungen an Verbindungen Mit P—NH—P-Gerüst/X-Ray Structural Studies on Compounds with a P-NH-P Backbone. Z. Naturforsch. B 1984, 39, 744–753. [Google Scholar] [CrossRef]
- Albert Cotton, F.; Kühn, F.E.; Yokochi, A. Synthesis and Structure of trans-[Di(μ-Acetato)Dichlorodi-(μ-Bis(Diphenylphosphino)Methylamine)Dimolybdenum(II)] and the Structure of Bis(Diphenylphosphino)Methylamine. Inorg. Chim. Acta 1996, 252, 251–256. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijn, J.; van Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua [1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Ewart, G.; Lane, A.P.; McKechnie, J.; Payne, D.S. Tervalent Phosphorus–Nitrogen Chemistry. Part II. Mono- and Bis-(Disphenylphosphino)Alkylamines. J. Chem. Soc. 1964, 1543–1547. [Google Scholar] [CrossRef]
- Bruker. APEX4; Bruker AXS Inc.: Madison, WI, USA, 2021. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
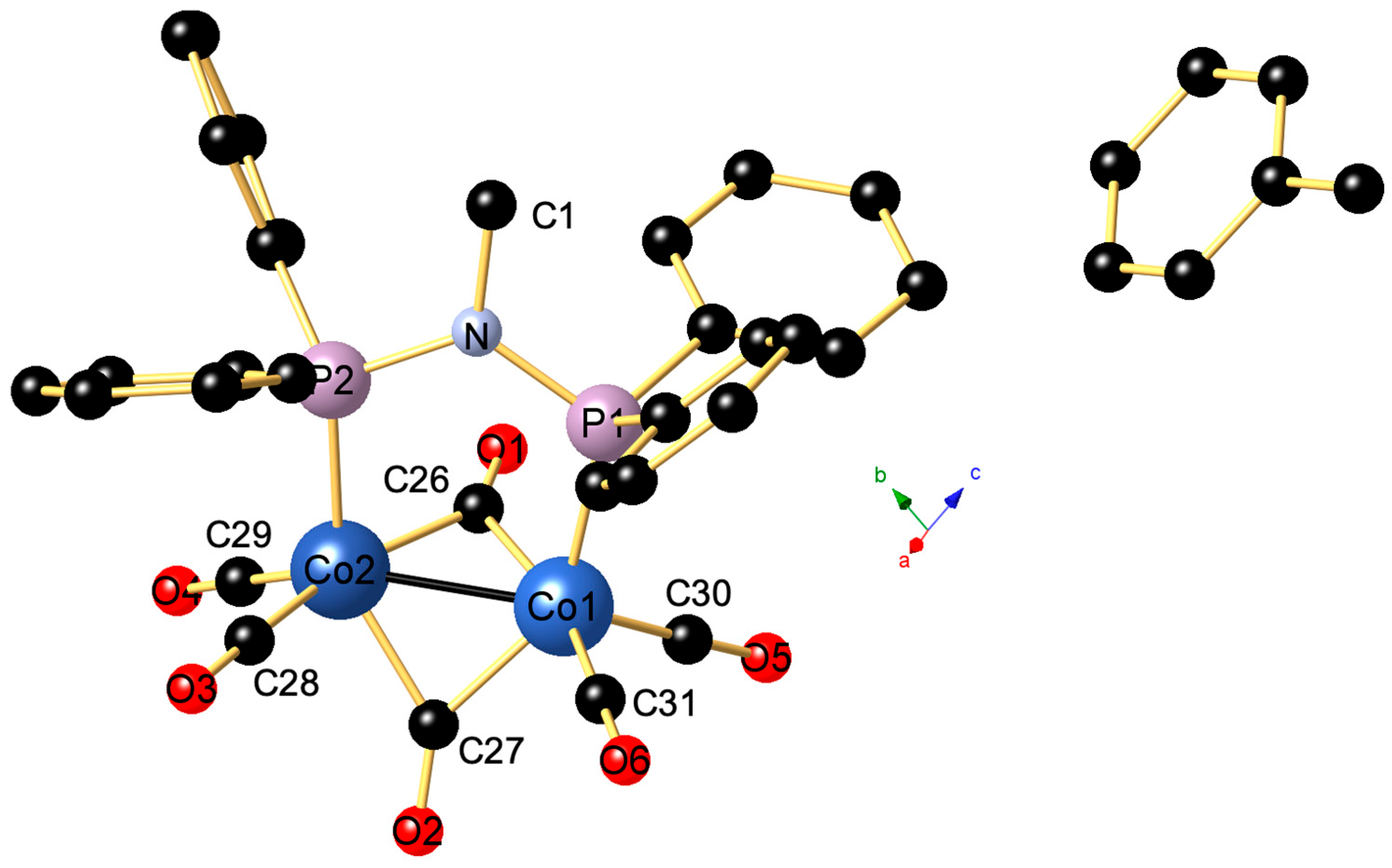
| 1 | dppa | dppm | dcpm | |
|---|---|---|---|---|
| Co–Co | 2.4595(5) | 2.4569 | 2.4569(4) | 2.4618(7) |
| Co–P | 2.234 | 2.229 | 2.252 | 2.262 |
| Co–μC | 1.939 | 1.914 | 1.938 | 1.927 |
| Co–C | 1.799 | 1.765 | 1.793 | 1.805 |
| P–X | 1.704 | 1.689 | 1.850 | 1.843 |
| PXP | 123.19(9) | 126.22 | 115.9(1) | 117.73(17) |
| CSD reference | This work | PPACCP [17] | JAZFUM [18] | PAJRUP [38] |
| 2 | iBudppa | dppm | dppm·2THF | |
|---|---|---|---|---|
| Co–C | 1.7441(19) | 1.796(9) | 1.735(12) | 1.786(7) |
| Co–P | 2.2224(5) | 2.179(2) | 2.217(3) | 2.195(2) |
| 2.2194(5) | 2.174(2) | 2.208(3) | 2.204(2) | |
| 2.1870(5) | 2.180(2) | 2.207(3) | 2.210(2) | |
| 2.1931(5) | 2.173(2) | 2.227(3) | 2.203(2) | |
| P–Co–C | 94.21(6) | 100.0(2) | 93.1(3) | 97.7(2) |
| 94.79(6) | 100.3(2) | 94.9(3) | 98.0(2) | |
| 122.31(7) | 103.8(2) | 113.8(4) | 104.9(2) | |
| 127.81(7) | 105.9(2) | 112.5(4) | 107.8(2) | |
| P–Co–P | 171.00(2) | 159.74(7) | 172.05(11) | 164.23(8) |
| 109.87(2) | 150.29(7) | 133.73(12) | 147.26(8) | |
| 102.36(2) | 102.24(6) | 102.53(11) | 99.84(8) | |
| 102.62(2) | 103.17(6) | 103.37(11) | 103.43(8) | |
| 72.10(2) | 71.94(6) | 73.73(11) | 73.83(8) | |
| 72.215(19) | 71.94(6) | 73.93(11) | 73.84(7) | |
| P–X–P | 100.42(9) | 97.3(2) | 92.6(5) | 93.0(3) |
| 99.88(8) | 96.8(2) | 92.4(4) | 92.9(3) | |
| CSD reference | This work | WEYJAL [33] | KOKTEJ [37] | LIQTAG [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Jourdain, I.; Knorr, M.; Strohmann, C.; Schmidt, A. Synthesis and Structural Characterization of Cobalt Complexes Ligated by N-Methyl(bis(diphenylphosphino)amine). Molbank 2025, 2025, M1967. https://doi.org/10.3390/M1967
Wang M, Jourdain I, Knorr M, Strohmann C, Schmidt A. Synthesis and Structural Characterization of Cobalt Complexes Ligated by N-Methyl(bis(diphenylphosphino)amine). Molbank. 2025; 2025(1):M1967. https://doi.org/10.3390/M1967
Chicago/Turabian StyleWang, Mélaine, Isabelle Jourdain, Michael Knorr, Carsten Strohmann, and Annika Schmidt. 2025. "Synthesis and Structural Characterization of Cobalt Complexes Ligated by N-Methyl(bis(diphenylphosphino)amine)" Molbank 2025, no. 1: M1967. https://doi.org/10.3390/M1967
APA StyleWang, M., Jourdain, I., Knorr, M., Strohmann, C., & Schmidt, A. (2025). Synthesis and Structural Characterization of Cobalt Complexes Ligated by N-Methyl(bis(diphenylphosphino)amine). Molbank, 2025(1), M1967. https://doi.org/10.3390/M1967






