1-(2,3,5,6-Tetramethylphenyl)ethan-1-one
Abstract
1. Introduction
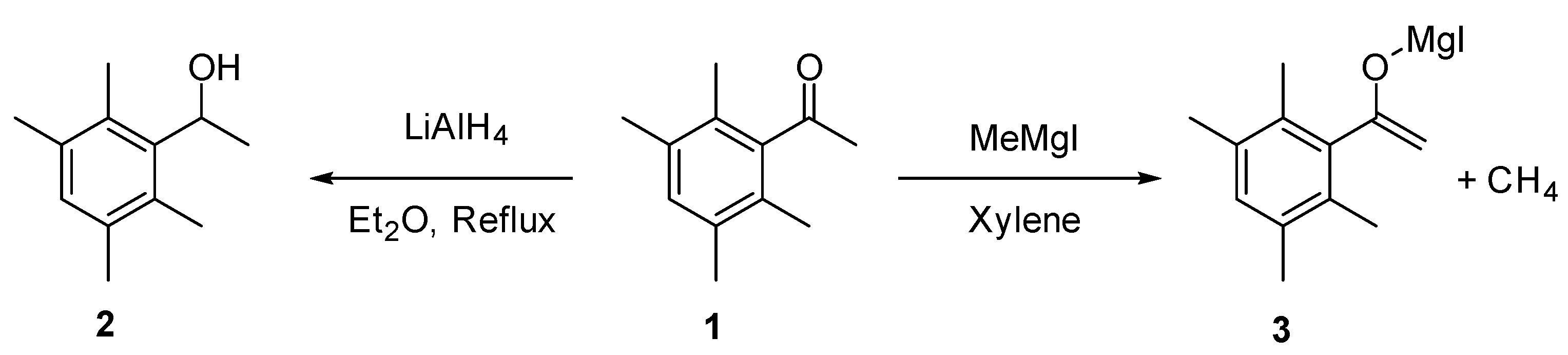
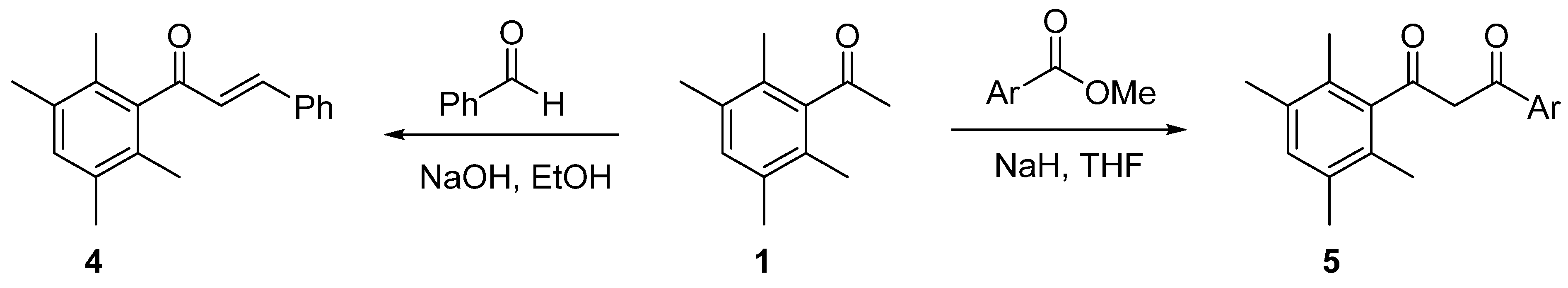
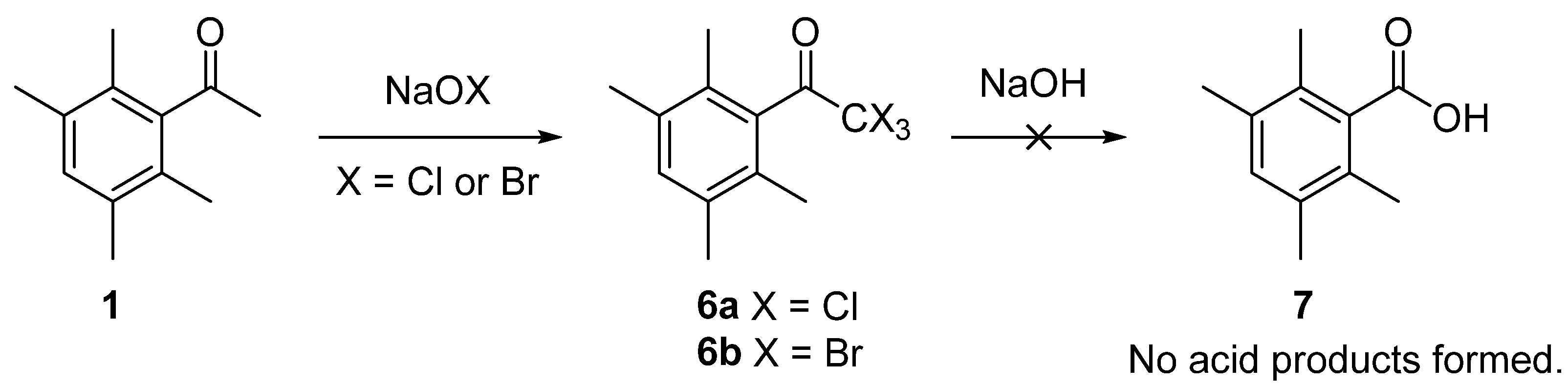
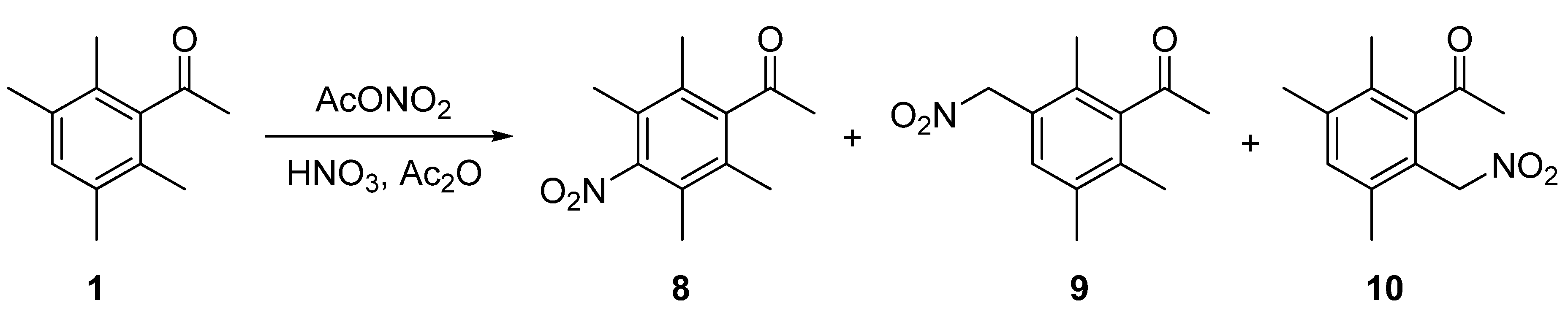
2. Results and Discussion
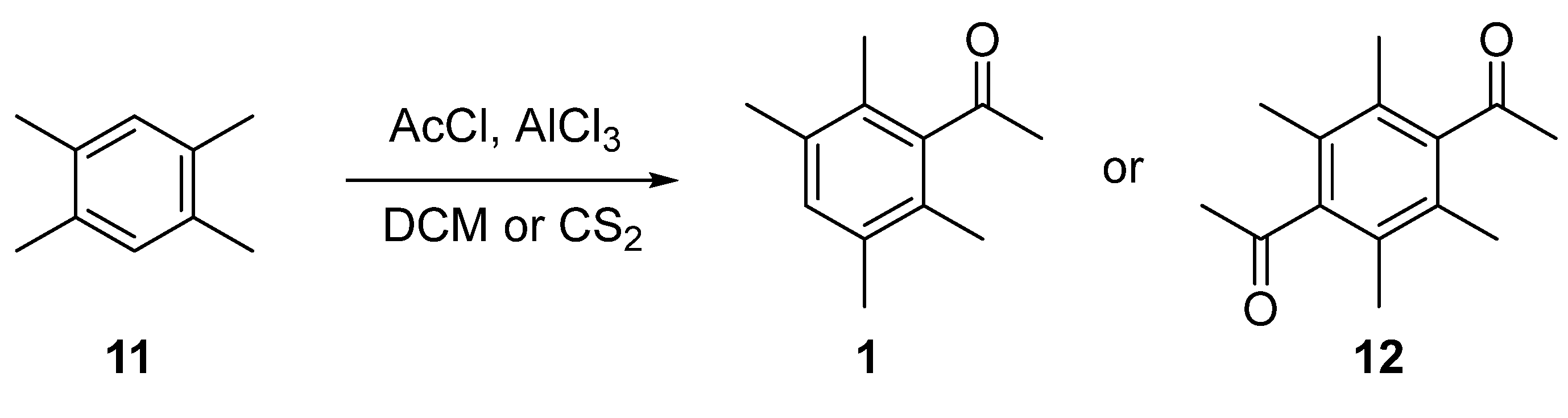
2.1. Synthesis of 1
2.2. NMR and IR Spectroscopy
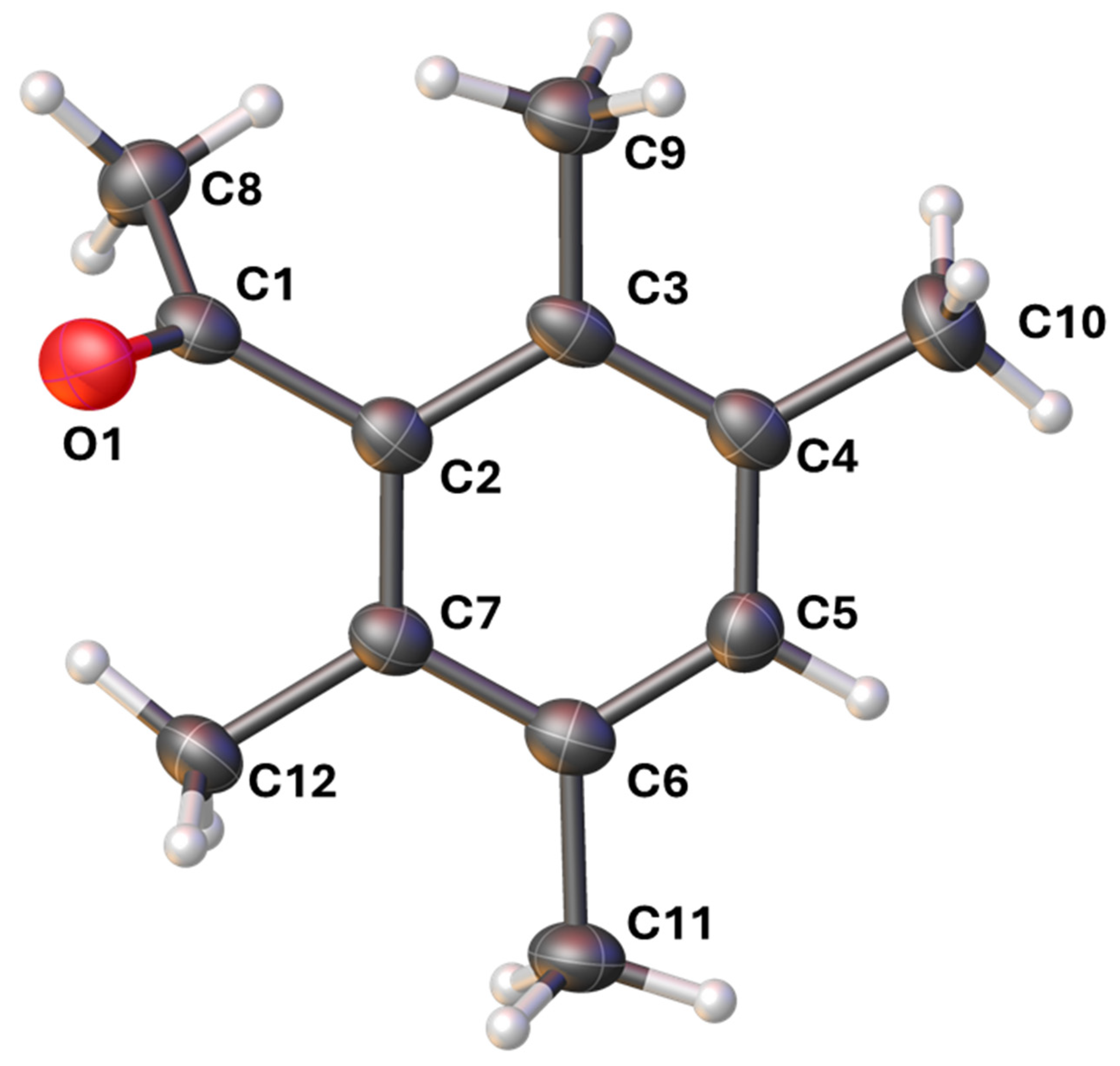
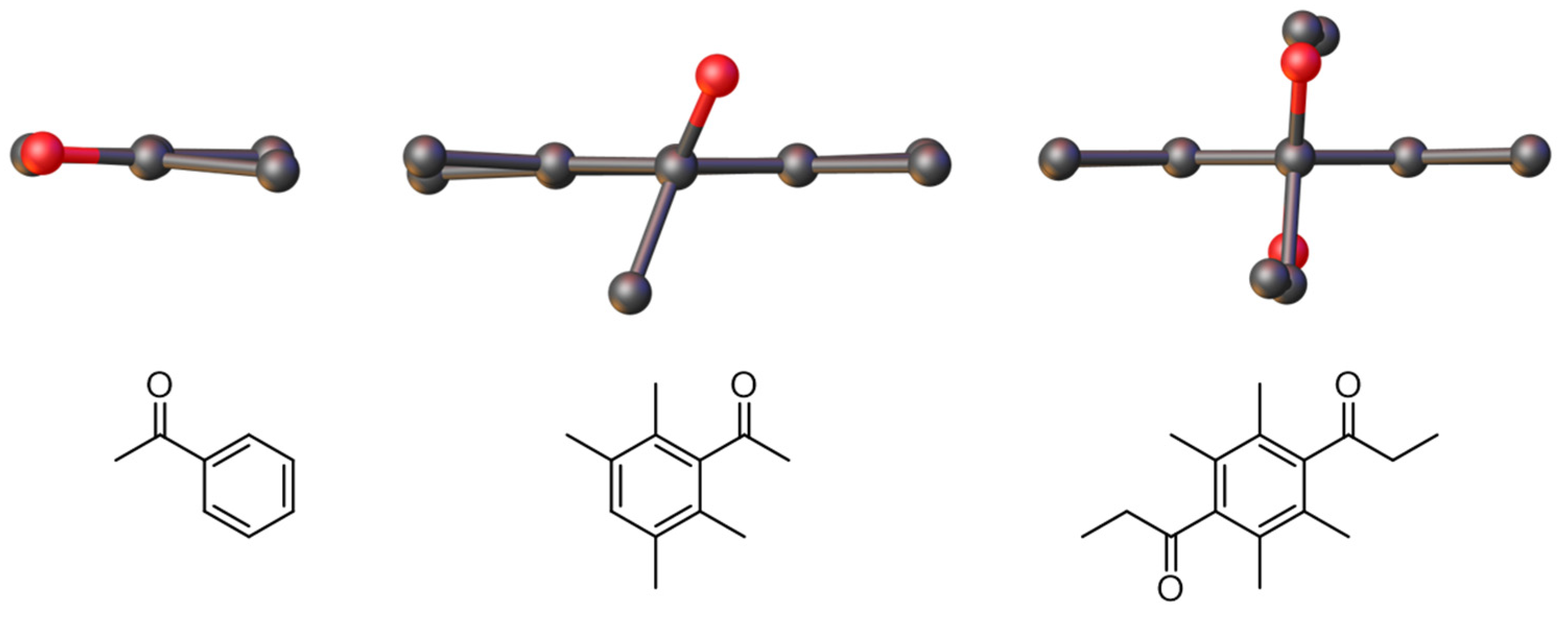
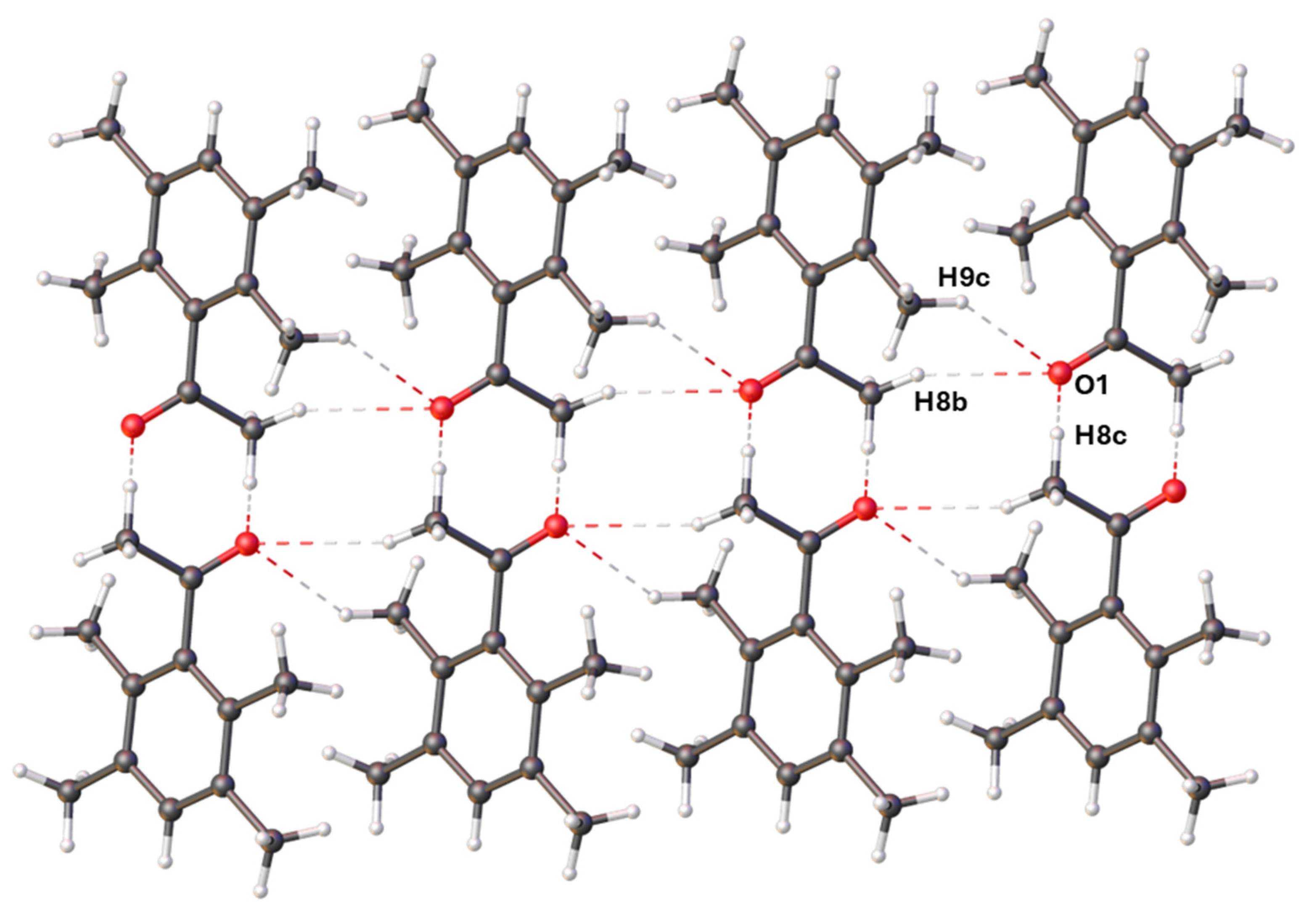
2.3. X-Ray Crystallography
3. Materials and Methods
3.1. Synthesis of 1-(2,3,5,6-Tetramethylphenyl)ethan-1-one (1)
3.2. X-Ray Crystallography Experimental Details
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, R.J.; Donohoe, T.J. Pentamethylphenyl (Ph*) ketones: Unique building blocks for organic synthesis. Tetrahedron Lett. 2021, 74, 153151–153159. [Google Scholar] [CrossRef]
- Lukin, M.; Corson, B.B. Preparation of Vinyltetramethylbenzenes. J. Org. Chem. 1958, 23, 1007–1009. [Google Scholar] [CrossRef]
- Smith, L.I.; Guss, C. The Enolizing Action of Methylmagnesium Iodide upon Hindered Ketones. J. Am. Chem. Soc. 1937, 59, 804–806. [Google Scholar] [CrossRef]
- Fuson, R.C.; Bannister, R.G. β-Duroylphenylpropionic Acids. J. Am. Chem. Soc. 1952, 74, 1629–1631. [Google Scholar] [CrossRef]
- Fuson, R.C.; Bannister, R.G. Regio- and Enantioselective Asymmetric Transfer Hydrogenation of One Carbonyl Group in a Diketone through Steric Hindrance. J. Org. Chem. 2024, 89, 2759–2763. [Google Scholar] [CrossRef]
- Gray, A.R.; Walker, J.T.; Fuson, R.C. The Haloform Reaction. III. Trihaloacetyl Derivatives of Mesitylene, Durene and Isodurene. J. Am. Chem. Soc. 1931, 53, 3494–3498. [Google Scholar] [CrossRef]
- Dambatta, M.B.; Santos, J.; Bolt, R.R.A.; Morrill, L.C. Transition metal free α-C-alkylation of ketones using secondary alcohols. Tetrahedron 2020, 76, 131571–131574. [Google Scholar] [CrossRef]
- Akhtar, W.M.; Cheong, C.B.; Frost, J.R.; Christensen, K.E.; Stevenson, N.G.; Donohoe, T.J. Hydogen Borrowing Catalysis with Secondary Alcohols: A New Route for the Generation of β-Branched Carbonyl Compounds. J. Am. Chem. Soc. 2017, 139, 2577–2580. [Google Scholar] [CrossRef]
- Keumi, T.; Morita, T.; Teramoto, K.; Takahashi, H.; Yamamoto, H.; Ikeno, K.; Hanaki, M.; Inagaki, T.; Kitajima, H. Regioselective side-chain nitration of polymethylbenzenes directed by an acyl function and its application to the synthesis of polysubstituted phthalic acid derivatives. J. Org. Chem. 1986, 51, 3439–3446. [Google Scholar] [CrossRef]
- Schlosberg, R.H.; Woodbury, R.P. Friedel-Crafts Isomerization of Tetramethylacetophenones. J. Org. Chem. 1972, 37, 2627–2629. [Google Scholar] [CrossRef]
- Hendy, B.N.; Patterson, K.H.; Smith, D.M.; Gardner, S.E.; Nicolson, N.J. Polyalkylated aromatic monomers and polymers. Part 1.-Friedel-Crafts diacylation of polymethylbenzenes as a means of monomer synthesis. J. Mater. Chem. 1995, 5, 199–204. [Google Scholar] [CrossRef]
- Andreou, A.D.; Bulbulian, R.V.; Gore, P.H. Friedel-Crafts Acetylation of Durene, Isodurene and Prehnitene. Tetrahedron 1980, 36, 2101–2104. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Kobayashi, H.; Nagakura, S.; Saito, Y. The crystal structure of acetophenone at 154 K. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 1822. [Google Scholar] [CrossRef]
- Pinkus, A.G.; Klausmeyer, K.K.; Carson, C.E.; Custard, H.C. Dipropiodurene. Acta Crystallogr. Sect. E 2006, 62, o1854. [Google Scholar] [CrossRef]
- CrysAlisPro, v1.171.42.109a; Rigaku Oxford Diffraction, Rigaku Corporation: Oxford, UK, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordes, D.B.; Smellie, I.A.; Chalmers, B.A. 1-(2,3,5,6-Tetramethylphenyl)ethan-1-one. Molbank 2025, 2025, M1961. https://doi.org/10.3390/M1961
Cordes DB, Smellie IA, Chalmers BA. 1-(2,3,5,6-Tetramethylphenyl)ethan-1-one. Molbank. 2025; 2025(1):M1961. https://doi.org/10.3390/M1961
Chicago/Turabian StyleCordes, David B., Iain A. Smellie, and Brian A. Chalmers. 2025. "1-(2,3,5,6-Tetramethylphenyl)ethan-1-one" Molbank 2025, no. 1: M1961. https://doi.org/10.3390/M1961
APA StyleCordes, D. B., Smellie, I. A., & Chalmers, B. A. (2025). 1-(2,3,5,6-Tetramethylphenyl)ethan-1-one. Molbank, 2025(1), M1961. https://doi.org/10.3390/M1961







