High-Yield Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine via N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine
Abstract
1. Introduction
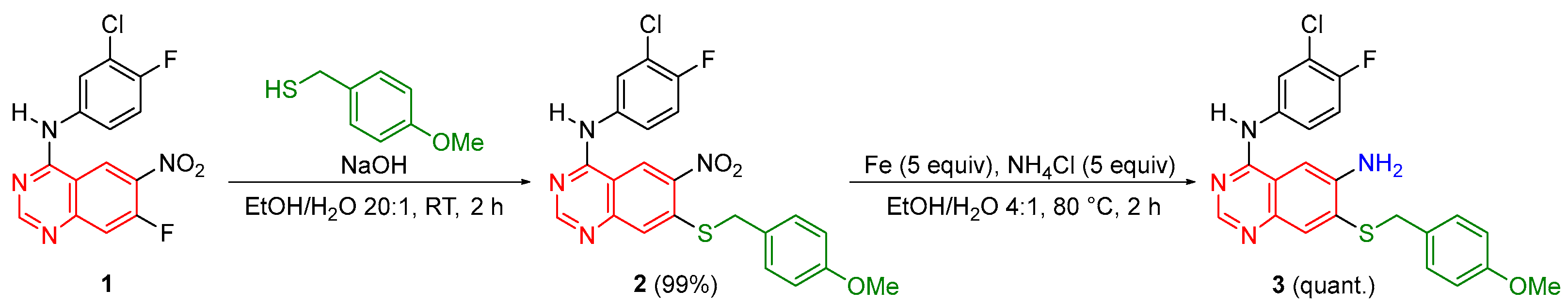
2. Results and Discussion
3. Materials and Methods
3.1. General Information and Analyses
3.2. Synthesis of N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine (2)
3.3. Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahekar, R.H.; Rao, A.R.R. Synthesis, Evaluation and Structure-Activity Relationships of 5-Alkyl-2,3-Dihydroimidazo[1,2-c]Quinazoline, 2,3-Dihydroimidazo[1,2-c]Quinazolin-5(6H)-Thiones and Their Oxo-Analogues as New Potential Bronchodilators. Arzneimittelforschung 2001, 51, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Špulák, M.; Pourová, J.; Vopršálová, M.; Mikušek, J.; Kuneš, J.; Vacek, J.; Ghavre, M.; Gathergood, N.; Pour, M. Novel Bronchodilatory Quinazolines and Quinoxalines: Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2014, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Chikhalia, K.H.; Kumari, P. Synthesis and Biological Evaluation of Novel Quinazoline Derivatives Obtained by Suzuki C–C Coupling. Med. Chem. Res. 2014, 23, 2338–2346. [Google Scholar] [CrossRef]
- Al-Salahi, R.; El-Tahir, K.-E.; Alswaidan, I.; Lolak, N.; Hamidaddin, M.; Marzouk, M. Biological Effects of a New Set 1,2,4-Triazolo[1,5-a]Quinazolines on Heart Rate and Blood Pressure. Chem. Cent. J. 2014, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I. An Insight into the Therapeutic Potential of Quinazoline Derivatives as Anticancer Agents. Med. Chem. Commun. 2017, 8, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, H.; Diab, A.; Al Musaimi, O. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer: Current Use and Future Prospects. Int. J. Mol. Sci. 2024, 25, 10008. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, R.; Piccirillo, M.C.; Sandomenico, C.; Carillio, G.; Montanino, A.; Daniele, G.; Giordano, P.; Bryce, J.; De Feo, G.; Di Maio, M.; et al. Gefitinib in Non Small Cell Lung Cancer. BioMed Res. Int. 2011, 2011, 815269. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sadhukhan, S.; Sonawane, A. 20 Years since the Approval of First EGFR-TKI, Gefitinib: Insight and Foresight. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188967. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Kies, M.S. ZD1839 (IressaTM) in Non-Small Cell Lung Cancer. Oncologist 2002, 7, 9–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, X.; Xiang, Y.; Fang, T.; Liu, J.; Lu, K. Afatinib for the Treatment of NSCLC with Uncommon EGFR Mutations: A Narrative Review. Curr. Oncol. 2023, 30, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V. Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. BioDrugs 2015, 29, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, Z.; Wang, L.; Yao, Y.; Liu, Y.; Hao, X.Z.; Wang, J.; Xing, P.; Li, J. Efficacy of Dacomitinib in Patients with EGFR-Mutated NSCLC and Brain Metastases. Thorac. Cancer 2021, 12, 3407–3415. [Google Scholar] [CrossRef]
- Shirley, M. Dacomitinib: First Global Approval. Drugs 2018, 78, 1947–1953. [Google Scholar] [CrossRef]
- Smaill, J.B.; Rewcastle, G.W.; Bridges, A.J.; Zhou, H.; Showalter, H.D.H.; Fry, D.W.; Nelson, J.M.; Sherwood, V.; Elliott, W.L.; Vincent, P.W.; et al. Tyrosine Kinase Inhibitors. 17. Irreversible Inhibitors of the Epidermal Growth Factor Receptor: 4-(Phenylamino)Quinazoline- and 4-(Phenylamino)Pyrido[3,2-d]Pyrimidine-6-Acrylamides Bearing Additional Solubilizing Functions. J. Med. Chem. 2000, 43, 1380–1397. [Google Scholar] [CrossRef] [PubMed]
- Ojida, A.; Ono, M.; Watari, K.; Shindo, N.; Fuchida, Y. Novel Compound Useful for Egfr Inhibition and Tumor Treatment. WO2018084321A1, 13 February 2020. [Google Scholar]
- Fakhoury, S.A.; Lee, H.T.; Reed, J.E.; Schlosser, K.M.; Sexton, K.E.; Tecle, H.; Winters, R.T. 4-Phenylamino-Quinazolin-6-Yl-Amides. US2005250761A1, 10 November 2005. [Google Scholar]
- Bhambra, A.; Weaver, G. 5,6,8-Trifluoroquinazolines to Treat Parasitic Infection. WO2024157021A1, 2 August 2024. [Google Scholar]
- Ardón-Muñoz, L.G.; Bolliger, J.L. Synthesis of Benzo[4,5]Thiazolo[2,3-c][1,2,4]Triazole Derivatives via C-H Bond Functionalization of Disulfide Intermediates. Molecules 2022, 27, 1464. [Google Scholar] [CrossRef]
- Ardón-Muñoz, L.G.; Bolliger, J.L. Synthesis of Sulfur Heterocycles by C–H Bond Functionalization of Disulfide Intermediates. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 507–512. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, S.; Bolliger, J.L. High-Yield Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine via N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine. Molbank 2025, 2025, M1958. https://doi.org/10.3390/M1958
Thapa S, Bolliger JL. High-Yield Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine via N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine. Molbank. 2025; 2025(1):M1958. https://doi.org/10.3390/M1958
Chicago/Turabian StyleThapa, Susila, and Jeanne L. Bolliger. 2025. "High-Yield Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine via N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine" Molbank 2025, no. 1: M1958. https://doi.org/10.3390/M1958
APA StyleThapa, S., & Bolliger, J. L. (2025). High-Yield Synthesis of N4-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)quinazoline-4,6-diamine via N-(3-Chloro-4-fluorophenyl)-7-((4-methoxybenzyl)thio)-6-nitroquinazolin-4-amine. Molbank, 2025(1), M1958. https://doi.org/10.3390/M1958






