9,10-Dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid
Abstract
1. Introduction
2. Results and Discussion
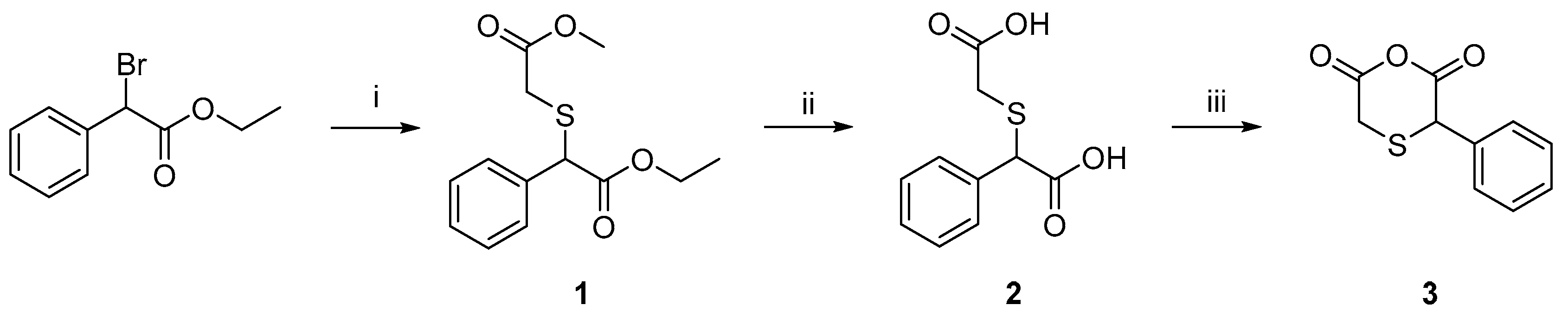
2.1. Preparation of 3-Phenyl-thiodiacetic Anhydride
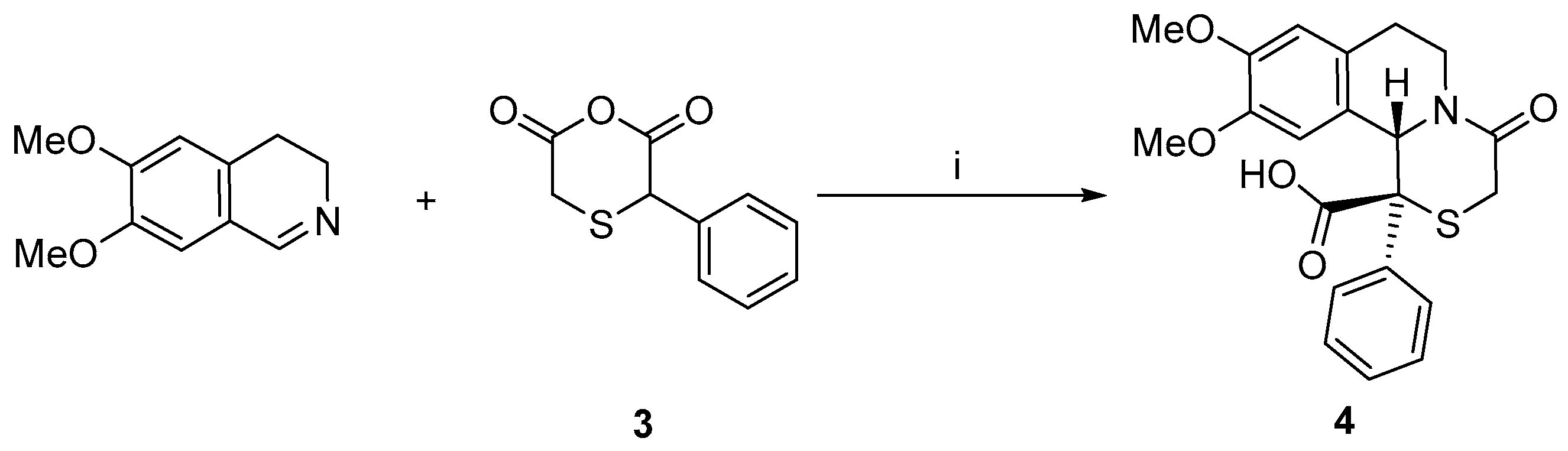
2.2. Reaction with 3,4-Dihydroisoquinoline and Its 1-Substituted Derivatives
3. Materials and Methods
3.1. Synthesis of 2-((Carboxymethyl)thio)-2-phenylacetic Acid 2
3.2. Synthesis of 3-Phenyl-1,4-oxathiane-2,6-dione 3
3.3. Synthesis of Rel-(1R,11bS)-9,10-dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid 4
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramiro, J.L.; Martínez-Caballero, S.; Neo, A.G.; Díaz, J.; Marcos, C.F. The Castagnoli-Cushman Reaction. Molecules 2023, 28, 2654. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, M.; Shaw, J.T. Cyclic Anhydrides in Formal Cycloadditions and Multicomponent Reactions. Chem. Rev. 2009, 109, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Krasavin, M.; Dar’in, D. Current diversity of cyclic anhydrides for the Castagnoli–Cushman-type formal cycloaddition reactions: Prospects and challenges. Tetrahedron Lett. 2016, 57, 1635. [Google Scholar] [CrossRef]
- Spanedda, M.V.; Bourel-Bonnet, L. Cyclic Anhydrides as Powerful Tools for Bioconjugation and Smart Delivery. Bioconjugate Chem. 2021, 32, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Shaw, J.T.; Younai, A. Anhydride-Based Multicomponent Reactions; Wiley-VCH Verlag Gmbh: Weinheim, Germany, 2015; pp. 379–400. [Google Scholar]
- Deshmukh, S.S.; Disale, S.T.; More, D.H.; Sapkal, B.M. One-pot Synthesis of Dihydrodibenzonaphthyridinediones through Sequential Imine Formation/Castagnoli-Cushman Reaction/Reductive Lactamization. Tetrahedron Lett. 2024, 135, 154885. [Google Scholar] [CrossRef]
- Lebedev, R.; Dar’in, D.; Kantin, G.; Bakulina, O.; Krasavin, M. One-Pot Sequence of Staudinger/aza-Wittig/Castagnoli–Cushman Reactions Provides Facile Access to Novel Natural-like Polycyclic Ring Systems. Molecules 2022, 27, 8130. [Google Scholar] [CrossRef] [PubMed]
- Lepikhina, A.; Bakulina, O.; Dar’in, D.; Krasavin, M. The first solvent-free synthesis of privileged gamma- and delta-lactams via the Castagnoli-Cushman reaction. RSC Adv. 2016, 6, 83808–83813. [Google Scholar] [CrossRef]
- Dar’in, D.; Bakulina, O.; Nikolskaya, S.; Gluzdikov, I.; Krasavin, M. The rare cis-configured trisubstituted lactam products obtained by the Castagnoli-Cushman reaction in N,N-dimethylformamide. RSC Adv. 2016, 6, 49411–49415. [Google Scholar] [CrossRef]
- Mohammadi, M.H.; Mohammadi, A.A. One-Pot, Three-Component Synthesis of Cis-Isoquinolonic Acids Using ZnCl2, AlCl3-SiO2 as Catalyst. Synth. Commun. 2011, 41, 523–527. [Google Scholar] [CrossRef]
- Karimi, A.R.; Momeni, H.R.; Pashazadeh, R. l-Proline-catalyzed diastereoselective synthesis of cis-isoquinolonic acids and evaluation of their neuroprotective effects. Tetrahedron Lett. 2012, 53, 3440–3443. [Google Scholar] [CrossRef]
- Ng, P.Y.; Masse, C.E.; Shaw, J.T. Cycloaddition Reactions of Imines with 3-Thiosuccinic Anhydrides: Synthesis of the Tricyclic Core of Martinellic Acid. Org. Lett. 2006, 8, 3999–4002. [Google Scholar] [CrossRef] [PubMed]
- Pashev, A.; Burdzhiev, N.; Stanoeva, E. One-step route to tricyclic fused 1,2,3,4-tetrahydroisoquinoline systems via the Castagnoli–Cushman protocol. Beilstein J. Org. Chem. 2020, 16, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Pashev, A.; Burdzhiev, N.; Stanoeva, E. Novel angularly substituted [1,4]thiazino[3,4-a]isoquinoline carboxylic acids prepared by cyclic imine-cyclic anhydride reaction. J. Heterocycl. Chem. 2023, 60, 513–518. [Google Scholar] [CrossRef]
- Pashev, A.; Petrov, V.; Pesheva, A.; Petrova, L.; Ilieva, K.; Stavreva, G.; Atanasova, M.; Cheshmedzhieva, D.; Altankov, G.; Aleksandrova, T. Angular-Substituted [1,4]Thiazino[3,4-a]Isoquinolines: Biological Evaluation and In Silico Studies on DPP-IV Inhibition. Int. J. Mol. Sci. 2024, 25, 11753. [Google Scholar] [CrossRef] [PubMed]
- Chizhova, M.; Bakulina, O.; Dar’in, D.; Krasavin, M. New Dicarboxylic Acid Anhydride for Ambient-Temperature Castagnoli-Cushman Reactions. ChemistrySelect 2016, 1, 5487–5492. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, V.; Aleksandrova, T.; Pashev, A. 9,10-Dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid. Molbank 2025, 2025, M1955. https://doi.org/10.3390/M1955
Petrov V, Aleksandrova T, Pashev A. 9,10-Dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid. Molbank. 2025; 2025(1):M1955. https://doi.org/10.3390/M1955
Chicago/Turabian StylePetrov, Valentin, Teodora Aleksandrova, and Aleksandar Pashev. 2025. "9,10-Dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid" Molbank 2025, no. 1: M1955. https://doi.org/10.3390/M1955
APA StylePetrov, V., Aleksandrova, T., & Pashev, A. (2025). 9,10-Dimethoxy-4-oxo-1-phenyl-1,3,4,6,7,11b-hexahydro-[1,4]thiazino[3,4-a]isoquinoline-1-carboxylic Acid. Molbank, 2025(1), M1955. https://doi.org/10.3390/M1955





