Catena-[Triaquabis(μ2-1,4-bis(diphenylphosphoryl)butane)nitrato-κ2O-praseodymium(III)] Nitrate Monohydrate Methanol Solvate
Abstract
1. Introduction
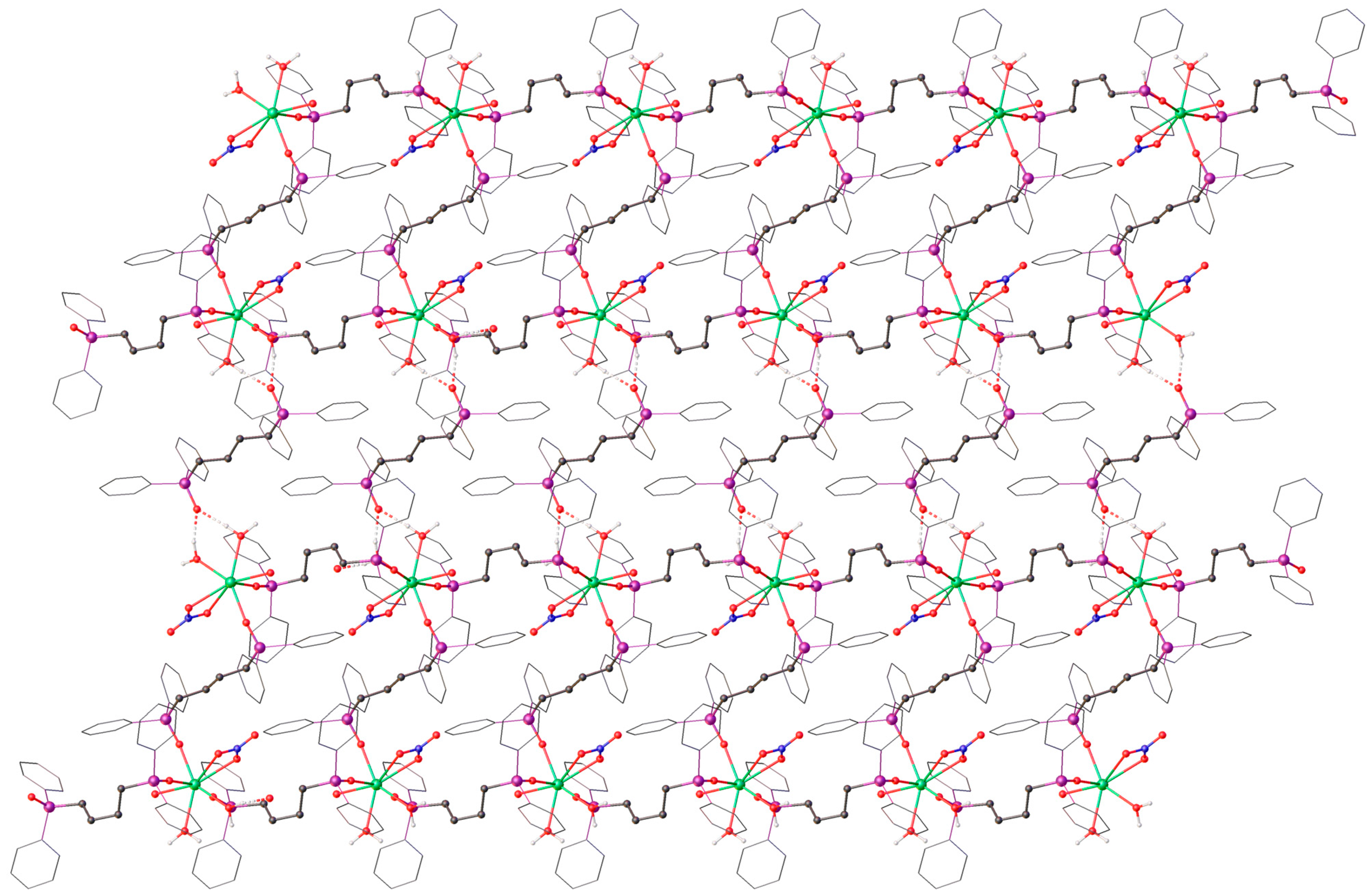
2. Results
3. Discussion
4. Materials and Methods
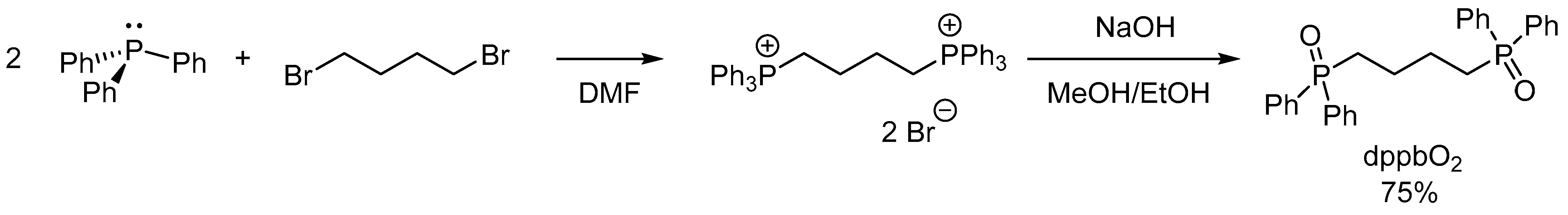
4.1. Synthesis of Bis(diphenylphosphoryl)butane (dppbO2)
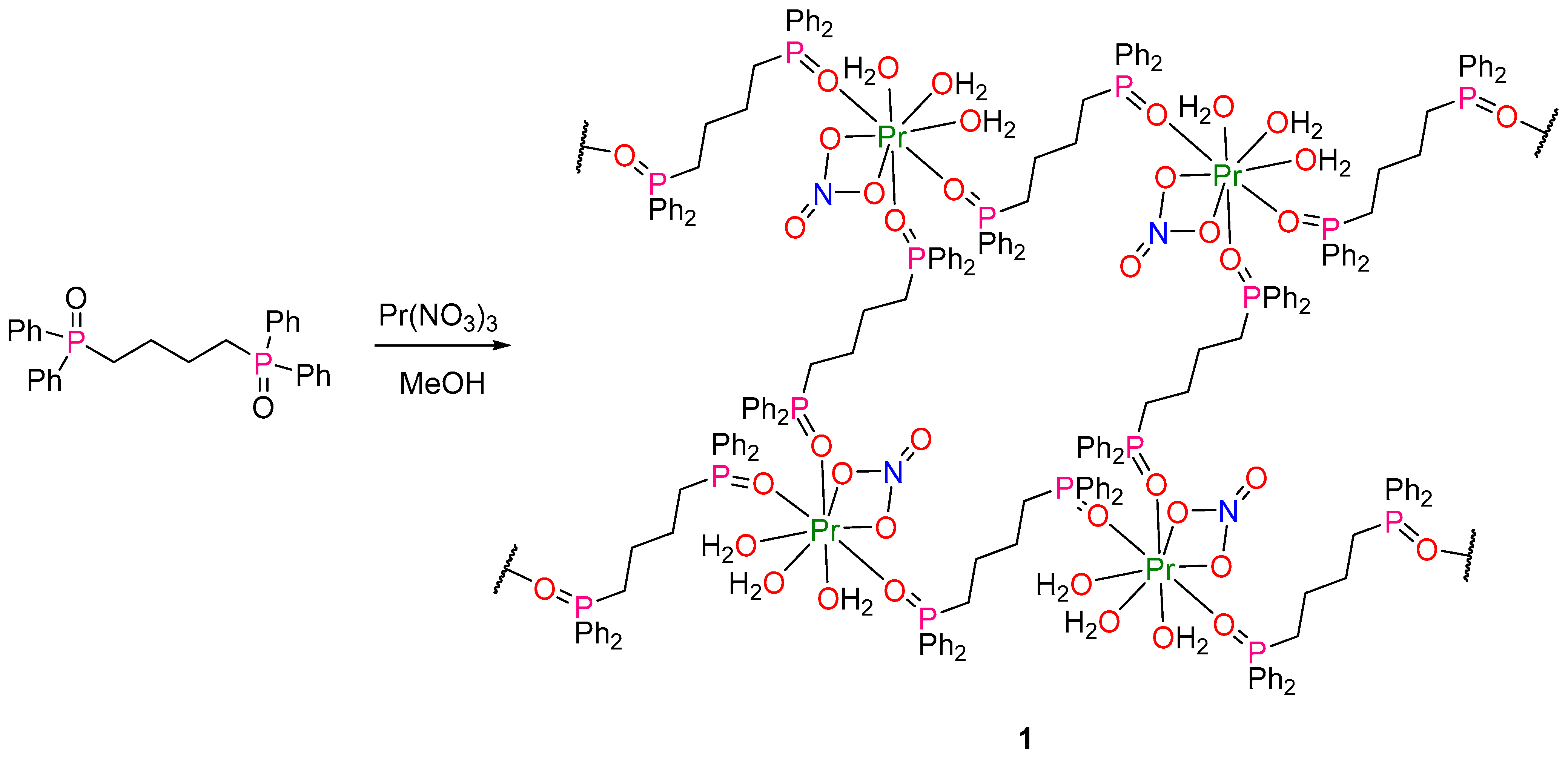
4.2. Synthesis of Catena-[{Pr(NO3)(H2O)3-μ-dppbO2}2-μ-dppbO2](NO3)4·(dppbO2)·2MeOH·2H2O
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, R.G.; Riebli, P. The Concept of P—H Protection Extended to Phosphine Oxides: Preparation of Functional, Unsymmetrical Secondary and Tertiary Phosphine Oxides. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1557–1562. [Google Scholar] [CrossRef]
- Abdel Nour, A.M.; Negm, N.A.; Sayed, G.H.; Tawfik, S.M.; Badr, E.A. Quantum Chemical and Electrochemical Evaluation of Alkyl Phosphine Oxide in Corrosion Inhibition of Carbon Steel in Formation Water. Z. Phys. Chem. 2019, 233, 1761–1785. [Google Scholar] [CrossRef]
- Benaglia, M.; Rossi, S. Chiral phosphine oxides in present-day organocatalysis. Org. Biomol. Chem. 2010, 8, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Ayad, T.; Gernet, A.; Pirat, J.-L.; Virieux, D. Enantioselective reactions catalysed by phosphine oxides. Tetrahedron 2019, 75, 4385–4418. [Google Scholar] [CrossRef]
- Sevrain, N.; Volle, J.-N.; Pirat, J.-L.; Ayad, T.; Virieux, D. 1,1′-Dibenzyl-bis-(triazolyl)diphenylphosphine dioxide: A new efficient organocatalyst for silicon tetrachloride-mediated enantioselective Abramov-type phosphonylation of aldehydes with trialkyl phosphites. RSC Adv. 2017, 7, 52101–52104. [Google Scholar] [CrossRef]
- Warner, C.J.A.; Berry, S.S.; Jones, S. Evaluation of bifunctional chiral phosphine oxide catalysis for the asymmetric hydrosilylation of ketimines. Tetrahedron 2019, 75, 130733. [Google Scholar] [CrossRef]
- Platt, A.W.G. Lanthanide phosphine oxide complexes. Coord. Chem. Rev. 2017, 340, 62–78. [Google Scholar] [CrossRef]
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical Applications of Lanthanide Nanomaterials, for Imaging, Sensing and Therapy. Nanotheranostics 2022, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M. Solvent extraction chemistry in ionic liquids: An overview of f-ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]
- Atanassova, M.; Kukeva, R.; Kurteva, V. New Sustainable Solvent Extraction Pathways for Rare Earth Metals via Oximes Molecules. Molecules 2023, 28, 7467. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, P.; Kariuki, B.M.; Kitchin, S.J.; Robinson, J.M.A.; Philp, D.; Harris, K.D.M. Understanding the Structural Properties of a Homologous Series of Bis-diphenylphosphine Oxides. Chem. Eur. J. 2000, 6, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Spichal, Z.; Necas, M.; Pinkas, J. From Parquets to Bricks: A Series of Lanthanide Coordination Polymers with Bis(diphenylphosphino)ethane Dioxide. Inorg. Chem. 2005, 44, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Spichal, Z.; Necas, M.; Pinkas, J.; Zdrahal, Z. Binuclear complexes of lanthanides with 1,4-bis(diphenylphosphino)butane dioxide. Polyhedron 2006, 25, 809–814. [Google Scholar] [CrossRef]
- Lees, A.M.J.; Platt, A.W.G. Complexes of Lanthanide Nitrate with Bis(diphenylphosphino)methane Dioxide. Inorg. Chem. 2003, 42, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Spichal, Z.; Necas, M.; Pinkas, J.; Novosad, J. The Synthesis and Crystal Structures of Two-Dimensional Coordination Polymers of Ph2P(O)−CH2CH2−P(O)Ph2 and Ph2P(O)-C5H3N-P(O)Ph2 with Praseodymium. Inorg. Chem. 2004, 43, 2776–2778. [Google Scholar] [CrossRef]
- McCann, B.W.; De Silva, N.; Windus, T.L.; Gordon, M.S.; Moyer, B.A.; Bryantsev, V.S.; Hay, B.P. Computer-Aided Molecular Design of Bis-phosphine Oxide Lanthanide Extractants. Inorg. Chem. 2016, 55, 5787–5803. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.E.; Reppart, W.J. The structure of the lanthanide ethyl sulfate enneahydrates, M(C2H5SO4)3·9H2O [M = La-Lu (except Pm)] at 171 K. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, C40, 781–786. [Google Scholar] [CrossRef]
- CrysAlisPro, v1.171.43.109a. Rigaku Oxford Diffraction. Rigaku Corporation: Oxford, UK, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
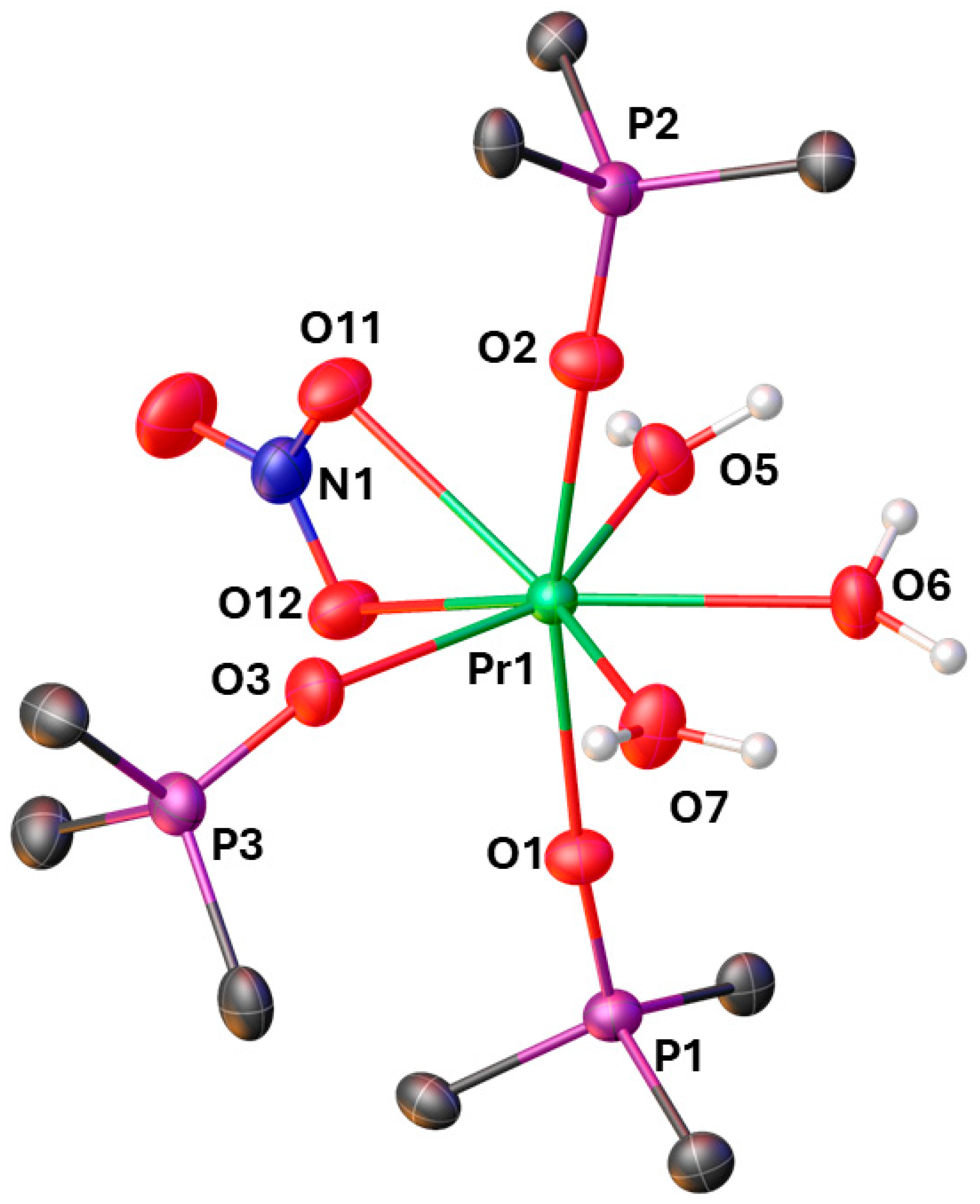
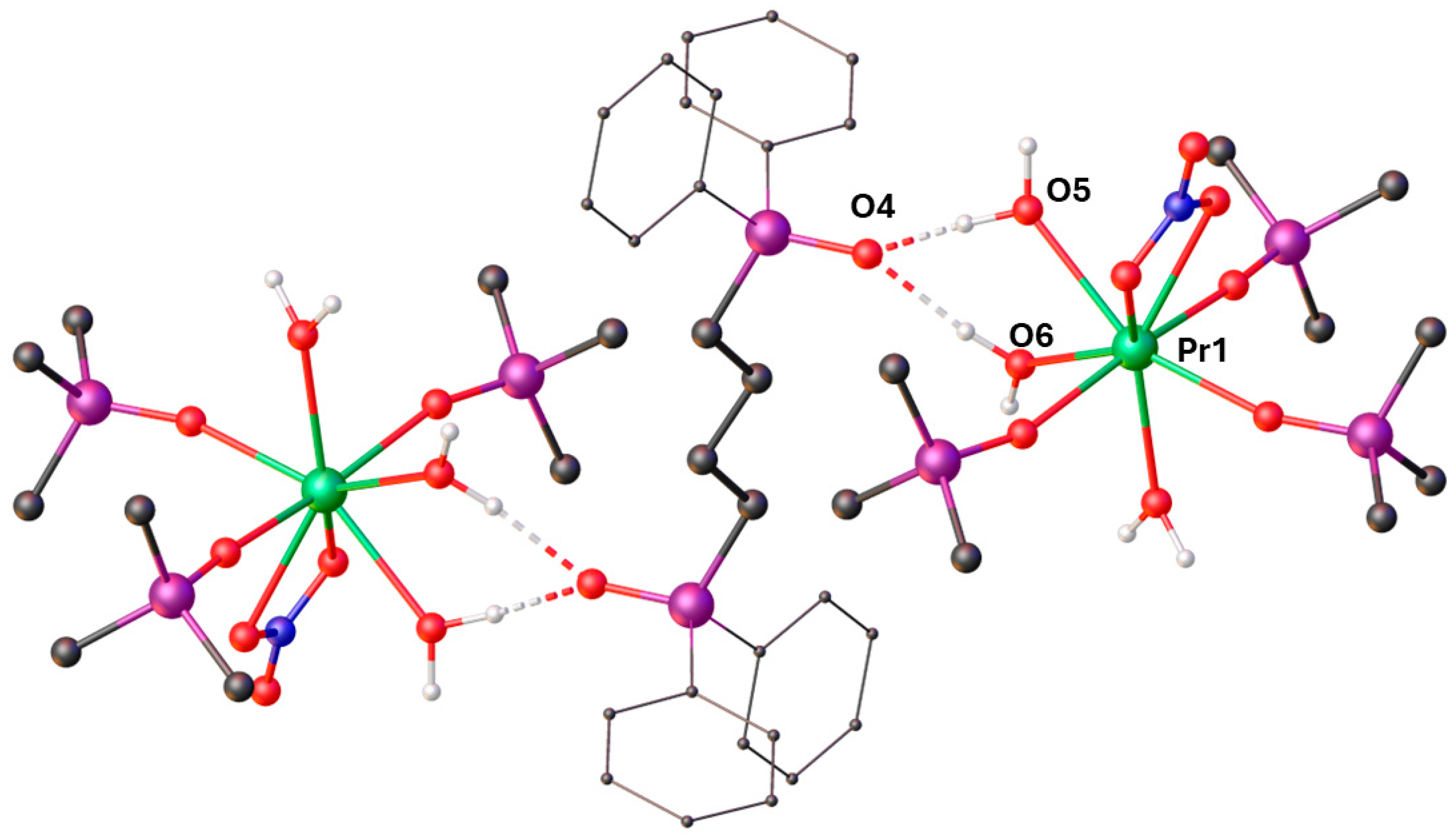
| Pr1–O1 | 2.358(5) | P1–O1 | 1.504(5) |
| Pr1–O2 | 2.358(5) | P2–O2 | 1.503(5) |
| Pr1–O3 | 2.389(3) | P3–O3 | 1.496(4) |
| Pr1–O5 | 2.492(3) | P4–O4 | 1.497(4) |
| Pr1–O6 | 2.499(4) | N1–O11 | 1.265(7) |
| Pr1–O7 | 2.482(4) | N1–O12 | 1.269(7) |
| Pr1–O11 | 2.597(4) | O4⋯H5b | 1.75(3) |
| Pr1–O12 | 2.588(4) | O4⋯H6b | 1.82(4) |
| O4⋯O5 | 2.688(5) | O4⋯O6 | 2.733(5) |
| O4⋯H6b | 1.82(4) | O4⋯H5b | 1.75(3) |
| O1–Pr1–O2 | 165.5(1) | O5–Pr1–O6 | 68.6(1) |
| O1–Pr–O3 | 89.7(1) | O6–Pr1–O7 | 70.6(1) |
| O2–Pr1–O3 | 95.7(1) | ||
| C29–C30–C30–C29 | 180.0(5) | C1–C2–C3–C4 | 71.1(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fern, E.S.M.; Lunt, M.I.; Minch, G.D.; Roeterdink, J.; Scheu Rodriguez, A.P.; Smith, C.A.; Venters, J.J.; McKay, A.P.; Cordes, D.B.; Chalmers, B.A. Catena-[Triaquabis(μ2-1,4-bis(diphenylphosphoryl)butane)nitrato-κ2O-praseodymium(III)] Nitrate Monohydrate Methanol Solvate. Molbank 2024, 2024, M1861. https://doi.org/10.3390/M1861
Fern ESM, Lunt MI, Minch GD, Roeterdink J, Scheu Rodriguez AP, Smith CA, Venters JJ, McKay AP, Cordes DB, Chalmers BA. Catena-[Triaquabis(μ2-1,4-bis(diphenylphosphoryl)butane)nitrato-κ2O-praseodymium(III)] Nitrate Monohydrate Methanol Solvate. Molbank. 2024; 2024(3):M1861. https://doi.org/10.3390/M1861
Chicago/Turabian StyleFern, Eilidh S. M., Maia I. Lunt, Guy D. Minch, Julia Roeterdink, Ana P. Scheu Rodriguez, Charlotte A. Smith, Johnathan J. Venters, Aidan P. McKay, David B. Cordes, and Brian A. Chalmers. 2024. "Catena-[Triaquabis(μ2-1,4-bis(diphenylphosphoryl)butane)nitrato-κ2O-praseodymium(III)] Nitrate Monohydrate Methanol Solvate" Molbank 2024, no. 3: M1861. https://doi.org/10.3390/M1861
APA StyleFern, E. S. M., Lunt, M. I., Minch, G. D., Roeterdink, J., Scheu Rodriguez, A. P., Smith, C. A., Venters, J. J., McKay, A. P., Cordes, D. B., & Chalmers, B. A. (2024). Catena-[Triaquabis(μ2-1,4-bis(diphenylphosphoryl)butane)nitrato-κ2O-praseodymium(III)] Nitrate Monohydrate Methanol Solvate. Molbank, 2024(3), M1861. https://doi.org/10.3390/M1861







