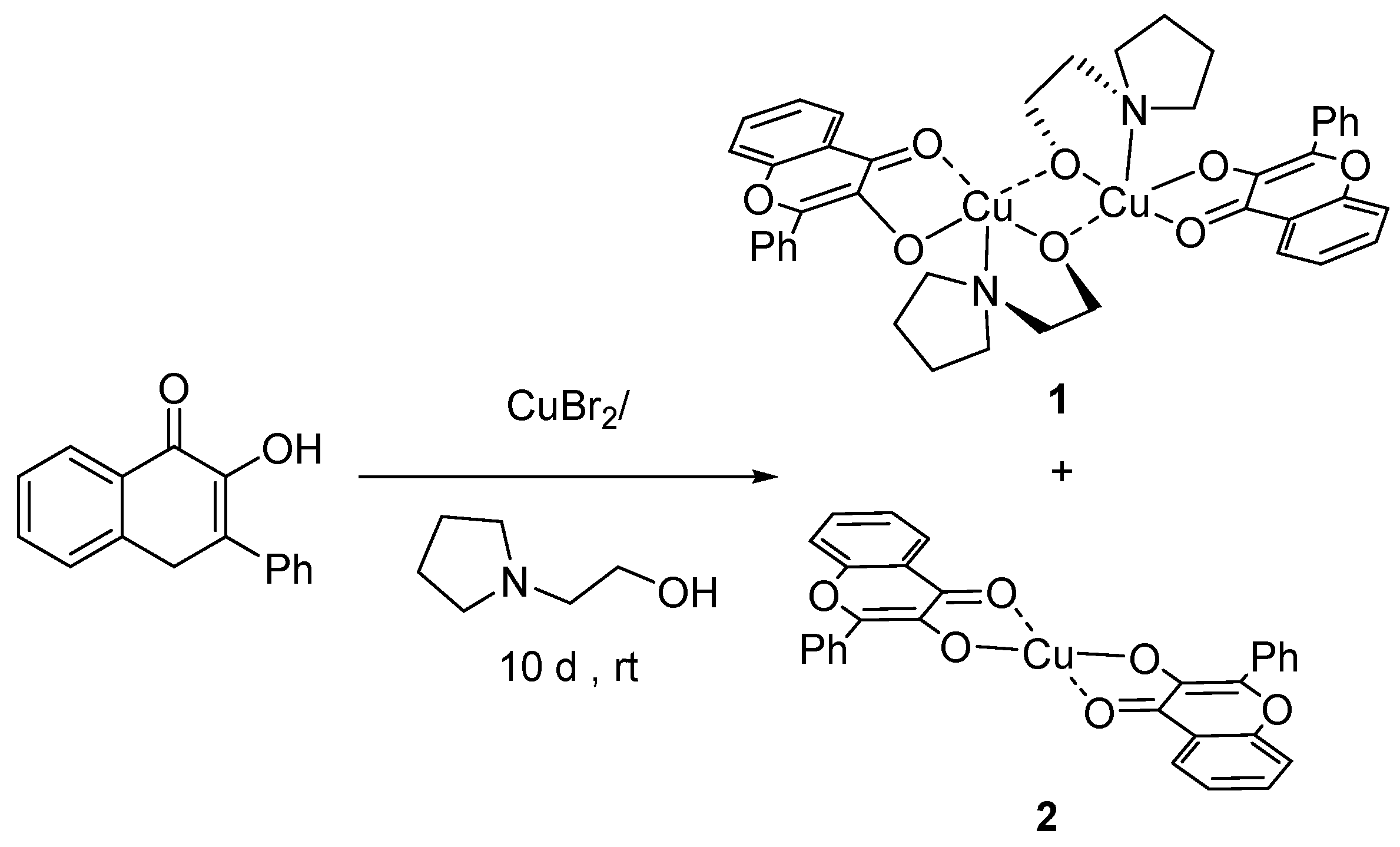
A (2-(Pyrrolidin-1-yl)ethan-1-olate)(1-oxo-3-phenyl-1,4-dihydronaphthalen-2-olate) μ-Oxo-Bridged Dicopper(II) Dimeric Complex
Abstract
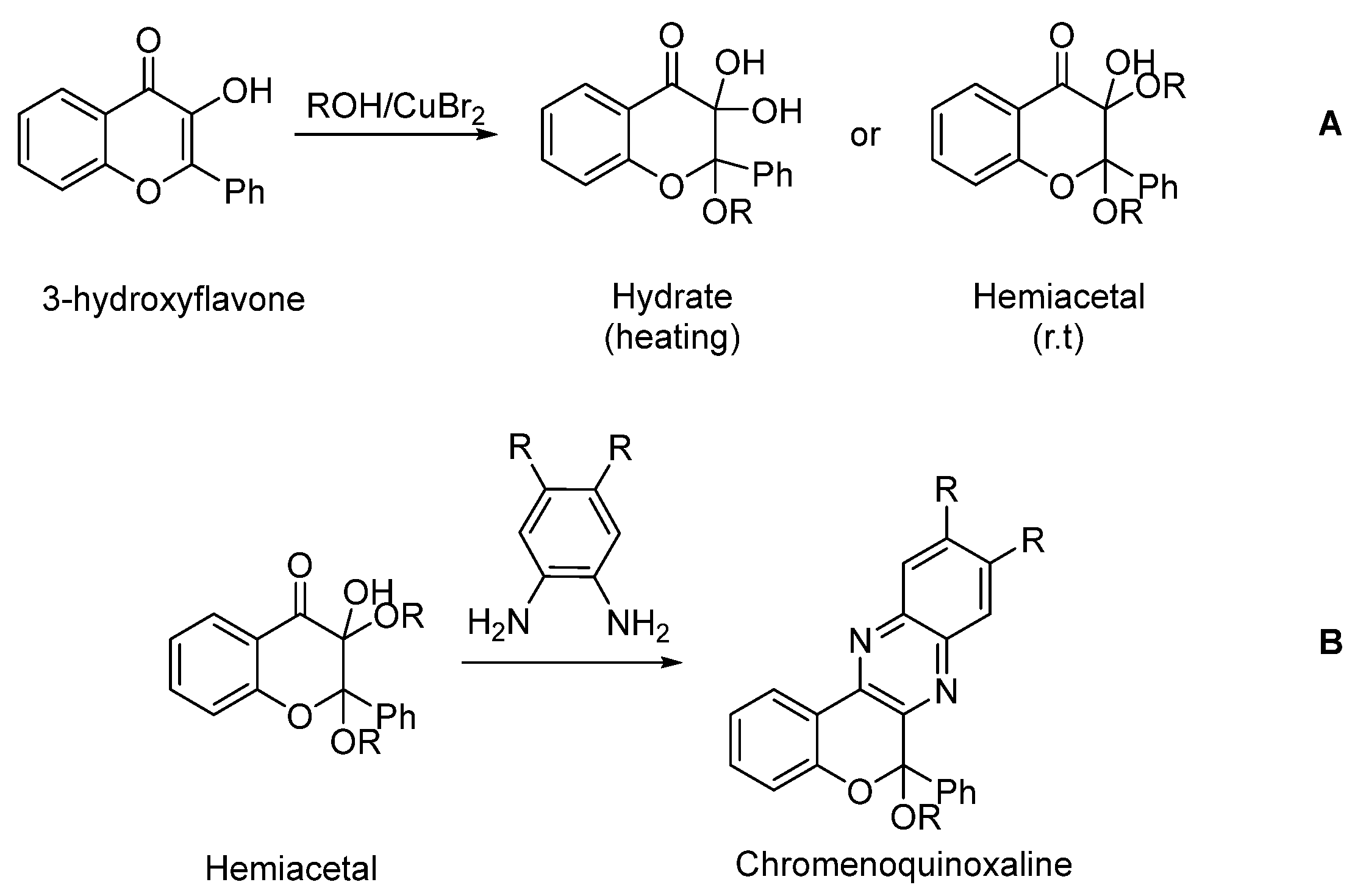
1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction Studies of 1
2.2. Spectroscopy of 1
3. Materials and Methods
3.1. General
3.2. Synthesis of 1 [μ-O-(κ2-O,O-flav)(κ2-N,O-2PEO)Cu]2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messerschmidt, A. 8.14—Copper Metalloenzymes. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 489–545. [Google Scholar] [CrossRef]
- Lewis, E.A.; Tolman, W.B. Reactivity of Dioxygen−Copper Systems. Chem. Rev. 2004, 104, 1047–1076. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.E.; Gagnon, N.L.; Neisen, B.D.; Dhar, D.; Spaeth, A.D.; Yee, G.M.; Tolman, W.B. Copper–Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity. Chem. Rev. 2017, 117, 2059–2107. [Google Scholar] [CrossRef] [PubMed]
- Lynch, W.E.; Nivens, D.; Quillian, B.; Padgett, C.W.; Petrillo, A.; Peek, N.; Stone, J. A Copper(II) tris-imidazolylphosphine complex as a functional model of flavonol 2,4-dioxygenase. J. Mol. Struct. 2019, 1185, 99–106. [Google Scholar] [CrossRef]
- Karlin, K.D.; Tyeklar, Z. Bioinorganic Chemistry of Copper; Springer: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Balogh-Hergovich, E.; Kaizer, J.; Speier, G. Studies of functional quercetinase models with copper and zinc ions. J. Inorg. Biochem. 1999, 74, 74. [Google Scholar]
- Balogh-Hergovich, E.; Kaizer, J.; Speier, G.; Argay, G.; Parkanyi, L. Kinetic studies on the copper(II)-mediated oxygenolysis of the flavonolate ligand. Crystal structures of [Cu(fla)(2)] (fla = flavonolate) and [Cu(O-bs)(2)(py)(3)] (O-bs = O-benzoylsalicylate). J. Chem. Soc. Dalton 1999, 3847–3854. [Google Scholar] [CrossRef]
- Balogh-Hergovich, E.; Kaizer, J.; Speier, G.; Fulop, V.; Parkanyi, L. Quercetin 2,3-dioxygenase mimicking ring cleavage of the flavonolate ligand assisted by copper. Synthesis and characterization of copper(I) complexes [Cu(PPh3)(2)(fla)] (fla = flavonolate) and [Cu(PPh3)(2)(O-bs)] (O-bs = O-benzoylsalicylate). Inorg. Chem. 1999, 38, 3787–3795. [Google Scholar] [CrossRef]
- Pap, J.S.; Kaizer, J.; Speier, G. Model systems for the CO-releasing flavonol 2,4-dioxygenase enzyme. Coord. Chem. Rev. 2010, 254, 781–793. [Google Scholar] [CrossRef]
- Wright, M.A.; Wright, J.A. PhotoCORMs: CO release moves into the visible. Dalton Trans. 2016, 45, 6801–6811. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Soboleva, T.; Arif, A.M.; Berreau, L.M. Properties of a flavonol-based photoCORM in aqueous buffered solutions: Influence of metal ions, surfactants and proteins on visible light-induced CO release. RSC Adv. 2017, 7, 21997–22007. [Google Scholar] [CrossRef]
- Beasley, E.M.; Bazemore, J.G.; Petrillo, A.; Padgett, C.W.; Lynch, W.E.; Quillian, B. Preparation of 3-hydroxy-2,3-dialkoxy-2-phenylchroman-4-ones and 3,3-dihydroxy-2-alkoxy-2-phenylchroman-4-ones by oxidation of 3-hydroxyflavone with copper(II) bromide: Structure, reactivity and characterization. Inorg. Chim. Acta 2020, 512, 119855. [Google Scholar] [CrossRef]
- Utaka, M.; Takeda, A. Copper(Ii)-Catalyzed Oxidation of Quercetin and 3-Hydroxyflavone. J. Chem. Soc. Chem. Commun. 1985, 1824–1826. [Google Scholar] [CrossRef]
- Dey, S.P.; Chattopadhyay, F.; MaIlik, A.K. Hypervalent iodine oxidation of flavonols and 3-hydroxy-2-styrylchromones in different alcohols. J. Indian Chem. Soc. 2016, 93, 1321–1324. [Google Scholar]
- Watkins, H.; Lee, G.; Ouedraogo, P.H.B.; Padgett, C.W.; Nguyen, K.; Artis, R.; Quillian, B.P. Condensation reactions of dialkoxy-2-phenylchroman-4-ones with 1,2-diamines: A method for the preparation of chromenoquinoxalines. Tetrahedron Lett. 2023, 132, 154820. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I.; Mathew, S.; Rahman, S. Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Med. Chem. 2020, 11, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Lainé, M.; Fanning, S.W.; Chang, Y.-F.; Green, B.; Greene, M.E.; Komm, B.; Kurleto, J.D.; Phung, L.; Greene, G.L. Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer. Breast Cancer Res. 2021, 23, 54. [Google Scholar] [CrossRef]
- Komm, B.S.; Mirkin, S. An overview of current and emerging SERMs. J. Steroid Biochem. Mol. Biol. 2014, 143, 207–222. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Seppälä, P.; Sillanpää, R.; Lehtonen, A. Structural diversity of copper(II) amino alcoholate complexes. Coord. Chem. Rev. 2017, 347, 98–114. [Google Scholar] [CrossRef]
- Wang, S. Polynuclear copper (II) complexes with aminoalcohol ligands. J. Clust. Sci. 1995, 6, 463–484. [Google Scholar] [CrossRef]
- Balogh-Hergovich, É.; Speier, G.; Argay, G. The oxygenation of flavonol by copper(I) and copper(II) flavonolate complexes. The crystal and molecular structure of bis(flavonolato)copper(II). J. Chem. Soc. Chem. Commun. 1991, 551–552. [Google Scholar] [CrossRef]
- Farrugia, L.J.; Middlemiss, D.S.; Sillanpää, R.; Seppälä, P. A Combined Experimental and Theoretical Charge Density Study of the Chemical Bonding and Magnetism in 3-Amino-propanolato Cu(II) Complexes Containing Weakly Coordinated Anions. J. Phys. Chem. A 2008, 112, 9050–9067. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Banci, L.; Bencini, A.; Dapporto, P.; Dei, A.; Gatteschi, D. Crystal and molecular structure and ESR spectra of a dimeric dialkoxo-bridged five-coordinate copper(II) complex. Inorg. Chem. 1980, 19, 3395–3399. [Google Scholar] [CrossRef]
- Zheng, J.C.; Rousseau, R.J.; Wang, S. Homonuclear copper complexes with multidentate amino alcohol ligands. Synthesis and characterization of a dicopper zwitterion, bis(1,3-bis(dimethylamino)-2-propanol)tetrachlorodicopper and a tricopper compound, bis(1,3-bis(dimethylamino)-2-propanolato)tetrachlorotricopper. Inorg. Chem. 1992, 31, 106–110. [Google Scholar] [CrossRef]
- Smolander, K. The Crystal Structure of Bis[iodo-mu-(2-diethylaminoethanolato-N,mu-O)copper(II)]. Acta Chem. Scand. 1981, 35, 815–819. [Google Scholar] [CrossRef]
- van Niekerk, J.N.; Schoening, F.R.L. A new type of copper complex as found in the crystal structure of cupric acetate, Cu2(CH3COO)4.2H2O. Acta Crystallogr. 1953, 6, 227–232. [Google Scholar] [CrossRef]
- Bertolotti, F.; Forni, A.; Gervasio, G.; Marabello, D.; Diana, E. Experimental and theoretical charge density of hydrated cupric acetate. Polyhedron 2012, 42, 118–127. [Google Scholar] [CrossRef]
- Parvarinezhad, S.; Salehi, M.; Kubicki, M.; Khaleghian, A. Unprecedented formation of a μ-oxobridged dimeric copper (II) complex: Evaluation of structural, spectroscopic, and electronic properties by using theoretical studies and investigations biological activity studies of new Schiff bases derived from pyrazolone. Appl. Organomet. Chem. 2021, 35, e6443. [Google Scholar] [CrossRef]
- Li, S.-J.; Liu, J.-S.; Guo, J.; Ji, L.-L.; Song, W.-D.; Ma, D.-Y. Construction of two Novel 2D Coordination Frameworks with the Ligand H3nbtc: Synthesis, Crystal Structures, and Luminescence. Z. Für Anorg. Und Allg. Chem. 2012, 638, 832–837. [Google Scholar] [CrossRef]
- Sun, Y.-J.; Huang, Q.-Q.; Zhang, J.-J. Set of Fe(II)-3-Hydroxyflavonolate Enzyme–Substrate Model Complexes of Atypically Coordinated Mononuclear Non-Heme Fe(II)-Dependent Quercetin 2,4-Dioxygenase. ACS Omega 2017, 2, 5850–5860. [Google Scholar] [CrossRef]
- Kaizer, J.; Balogh-Hergovich, É.; Czaun, M.; Csay, T.; Speier, G. Redox and nonredox metal assisted model systems with relevance to flavonol and 3-hydroxyquinolin-4(1H)-one 2,4-dioxygenase. Coord. Chem. Rev. 2006, 250, 2222–2233. [Google Scholar] [CrossRef]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Ganeshpandian, M.; Sanjeev, A.; Tamilarasan, A.; Mattaparthi, V.S.K.; Islam, N.S.; Palaniandavar, M. Bis- and mixed-ligand copper(II) complexes of nalidixic acid the antibacterial drug: Mode of nalidixate coordination determines DNA binding and cleavage and cytotoxicity. Inorg. Chim. Acta 2020, 504, 119450. [Google Scholar] [CrossRef]
- Ajaykamal, T.; Köckerling, M.; Palaniandavar, M. Copper(II)-flavonolate complexes of 2N ligands as functional models for quercetin 2,4-dioxygenase enzymes: The role of axially coordinated water and ligand substitution on dioxygenase activity. Inorg. Chim. Acta 2023, 556, 121673. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 65th ed.; CRC Press: Boca Raton, FL, USA, 1984.
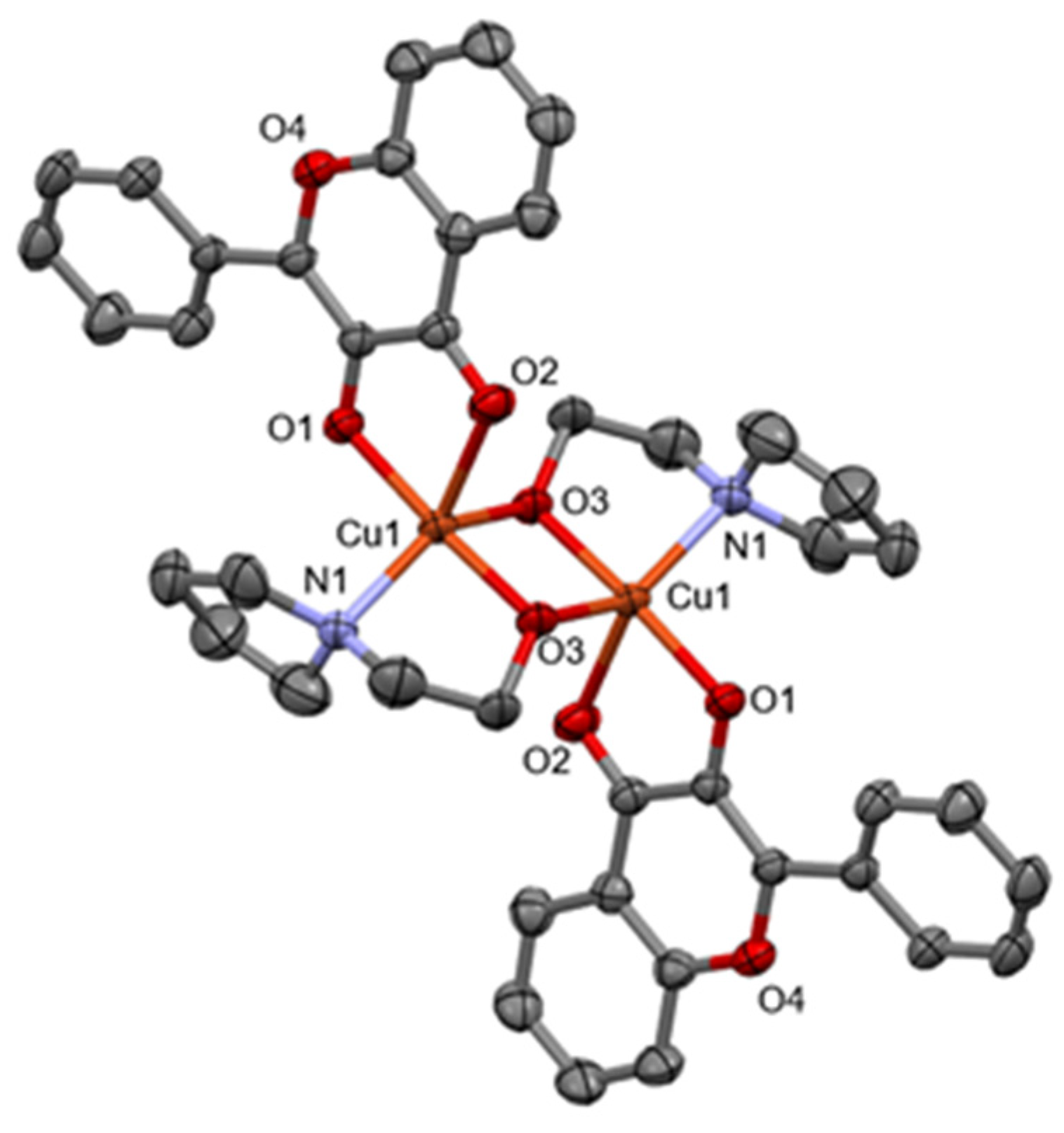
| Bonds | Distances | Atoms | Angles |
|---|---|---|---|
| Cu1‧‧‧Cu1 | 2.971 | Cu–O3–Cu1 | 98.44(8) |
| Cu1–O1 | 1.918(2) | O1–Cu1–O2 | 79.91(7) |
| Cu1–O2 | 2.247(2) | O1–Cu1–O3 | 175.51(8) |
| Cu1–O3 | 1.918(2) | O1–Cu1–O3 | 98.73(7) |
| Cu1–O3 | 2.004(2) | O1–Cu1–N1 | 96.8(2) |
| Cu1–N1 | 2.073(7) | O2–Cu1–O3 | 98.00(7) |
| O1–C2 | 1.313(3) | O2–Cu1–N1 | 117.6(1) |
| O2–C3 | 1.249(3) | O3–Cu1–N1 | 85.5(2) |
| O3–Cu1–O3 | 81.56(7) | ||
| N1–Cu1–O3 | 143.1(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artis, R.; Padgett, C.W.; Musso, K.; Shank, N.; Marks, A.; Quillian, B. A (2-(Pyrrolidin-1-yl)ethan-1-olate)(1-oxo-3-phenyl-1,4-dihydronaphthalen-2-olate) μ-Oxo-Bridged Dicopper(II) Dimeric Complex. Molbank 2024, 2024, M1846. https://doi.org/10.3390/M1846
Artis R, Padgett CW, Musso K, Shank N, Marks A, Quillian B. A (2-(Pyrrolidin-1-yl)ethan-1-olate)(1-oxo-3-phenyl-1,4-dihydronaphthalen-2-olate) μ-Oxo-Bridged Dicopper(II) Dimeric Complex. Molbank. 2024; 2024(3):M1846. https://doi.org/10.3390/M1846
Chicago/Turabian StyleArtis, Rylan, Clifford W. Padgett, Kennedy Musso, Nathaniel Shank, Allison Marks, and Brandon Quillian. 2024. "A (2-(Pyrrolidin-1-yl)ethan-1-olate)(1-oxo-3-phenyl-1,4-dihydronaphthalen-2-olate) μ-Oxo-Bridged Dicopper(II) Dimeric Complex" Molbank 2024, no. 3: M1846. https://doi.org/10.3390/M1846
APA StyleArtis, R., Padgett, C. W., Musso, K., Shank, N., Marks, A., & Quillian, B. (2024). A (2-(Pyrrolidin-1-yl)ethan-1-olate)(1-oxo-3-phenyl-1,4-dihydronaphthalen-2-olate) μ-Oxo-Bridged Dicopper(II) Dimeric Complex. Molbank, 2024(3), M1846. https://doi.org/10.3390/M1846







