Chlorido-(η6-p-cymene)-(bis(pyrazol-1-yl)methane-κ2N,N′)Osmium(II) Tetrafluoroborate, C17H22BClF4N4Os
Abstract
:1. Introduction
2. Results and Discussion
3. Discussion
4. Materials and Methods
4.1. Synthetic Procedure for 1
4.2. Spectroscopic Characterization of 1
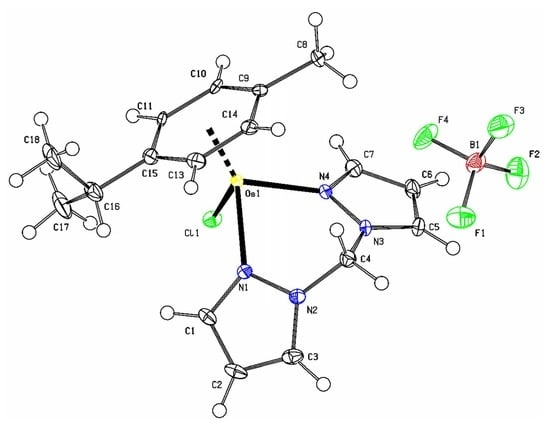
4.3. X-ray Diffraction Analysis of 1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nahari, G.; Tshuva, E.Y. Synthesis of asymmetrical diaminobis (alkoxo)-bisphenol compounds and their C1-symmetrical mono-ligated titanium(IV) complexes as highly stable highly active antitumor compounds. Dalton Trans. 2021, 50, 6423–6426. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.H.; Raninga, P.V.; Nakamura, K.; Casey, M.; Khanna, K.K.; Berners-Price, S.J.; Di Trapani, G.; Tonissen, K.F. Anticancer activity of a Gold(I) phosphine thioredoxin reductase inhibitor in multiple myeloma. Redox Biol. 2020, 28, 101310–101321. [Google Scholar] [CrossRef] [PubMed]
- Nabiyeva, T.; Marschner, C.; Blom, B. Synthesis, structure and anti-cancer activity of osmium complexes bearing π-bound arene substituents and phosphane Co-Ligands: A review. Eur. J. Med. Chem. 2020, 201, 112483–112498. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Hun, L.T. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Simović, A.R.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 113011–113036. [Google Scholar] [CrossRef]
- Hanif, M.; Babak, M.V.; Hartinger, C.G. Development of anticancer agents: Wizardry with osmium. Drug Discov. Today 2014, 19, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.; Sadler, P.J. Medicinal organometallic chemistry: Designing metal arene complexes as anticancer agents. Chem. Asian J. 2008, 3, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, H. Future potential of osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes. Dalton Trans. 2018, 47, 14841–14854. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platinum Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Păunescu, E.; Nowak-Sliwinska, P.; Clavel, C.M.; Scopelliti, R.; Griffioen, A.W.; Dyson, P.J. Anticancer Organometallic Osmium(II)-p-cymene Complexes. ChemMedChem 2015, 10, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.; Habtemariam, A.; Moggach, S.A.; Prescimone, A.; Parsons, S.; Sadler, P.J. Chloro half-sandwich osmium(II) complexes: Influence of chelated N, N-ligands on hydrolysis, guanine binding, and cytotoxicity. Inorg. Chem. 2007, 46, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, S.H.; Peacock, A.F.; Johnstone, R.D.; Parsons, S.; Sadler, P.J. Organometallic osmium (II) arene anticancer complexes containing picolinate derivatives. Inorg. Chem. 2009, 48, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Gichumbi, J.M.; Omondi, B.; Friedrich, H.B. Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V),) C19H26IN2F6Ru. Z. Krist. New Cryst. Struct. 2020, 235, 485–487. [Google Scholar] [CrossRef]
- Thangavel, S.; Rajamanikandan, R.; Friedrich, H.B.; Ilanchelian, M.; Omondi, B. Binding interaction, conformational change, and molecular docking study of N-(pyridin-2-ylmethylene) aniline derivatives and carbazole Ru (II) complexes with human serum albumins. Polyhedron 2016, 107, 124–135. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Omondi, B.; Friedrich, H.B. Crystal structure of (η6-1-isopropyl-4-methyl benzene)-(N-(2,5-dichlorophenyl)-1-(pyridin-2-yl)methanimine-k2N,N′)ruthenium(II) perchlorate, C22H22Cl4N2O4Ru. Z. Kristallogr. NCS 2018, 233, 423–425. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R.; Cerquetella, A.; Di Nicola, C.; Macchioni, A.; Zuccaccia, D.; Monari, M.; Piccinelli, F. Synthesis and intramolecular and interionic structural characterization of half-sandwich (arene) ruthenium(II) derivatives of bis (pyrazolyl) alkanes. Inorg. Chem. 2008, 47, 11593–11603. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B. Crystal structure of chlorido-(η6–1-isopropyl-4-methylbenzene)-(1-(pyridin-2-yl)-N-(p-tolyl)methanimine-κ2N,N′)tuthenium(II) hexafluorophosphate(V) C23H26ClF6N2PRu. Z. Krist. New Cryst. Struct. 2017, 232, 285–287. [Google Scholar] [CrossRef]
- Schreiber, D.F.; O’Connor, C.; Grave, C.; Müller-Bunz, H.; Scopelliti, R.; Dyson, P.J.; Phillips, A.D. Synthesis, Characterization, and Reactivity of the First Osmium β-Diketiminato Complexes and Application in Catalysis. Organometallics 2013, 32, 7345–7356. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B.; Naicker, K.; Singh, M.; Chenia, H.Y. Synthesis, characterization, antiproliferative, and antimicrobial activity of osmium(II) half-sandwich complexes. J. Coord. Chem. 2018, 71, 342–354. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CCD CrysAlis. CrysAlis Red; Xcalibur PX Software; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Bruker, A. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]




| Identification code for 1 | cu_am_ro_am_10_5_0m |
| Crystal (Colour /Shape) | Yellow /Block |
| Formula Weight | 594.84 |
| Temperature (K) | 102.22 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a (Å) | 27.4619(7) |
| b (Å) | 10.2573(3) |
| c (Å) | 14.4399(4) |
| α (°) | 90 |
| β (°) | 100.8160(10) |
| Γ (°) | 90 |
| Volume (Å3) | 3995.24(19) |
| Z | 8 |
| ρcalc, g/cm3 | 1.978 |
| Μ (mm−1) | 13.718 |
| F(000) | 2288 |
| Crystal size (mm3) | 0.475 × 0.33 × 0.15 |
| Radiation source, λ (Å) | Cu(Kα), λ = 1.54178 |
| 2θ range for data collection (°) | 6.554 to 136.734 |
| Index ranges | −32 ≤ h ≤ 32, −12 ≤ k ≤ 12, −17 ≤ l ≤ 16 |
| Reflections collected | 17267 |
| Independent reflections | 3581 [Rint = 0.0446, Rσ = 0.0383] |
| Data/restraints/parameters | 3581/0/256 |
| Goodness-of-fit on F2 | 1.245 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0275, wR2 = 0.0659 |
| Final R indexes [all data] | R1 = 0.0277, wR2 = 0.0661 |
| Largest diff. peak/hole / e Å−3 | 0.70/−1.68 |
| Length, (Å) | |
| Os1-Cl1 | 2.3982(8) |
| Os1-N1 | 2.122(3) |
| Os1-N4 | 2.105(3) |
| Angles/° | |
| N4-Os1-Cl1 | 84.71(9) |
| N4-Os1-N1 | 83.17(11) |
| N1-Os1-Cl1 | 83.84(9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambanda, A.; Kanyora, A.K.; Ongoma, P.; Gichumbi, J.; Omondi, R.O. Chlorido-(η6-p-cymene)-(bis(pyrazol-1-yl)methane-κ2N,N′)Osmium(II) Tetrafluoroborate, C17H22BClF4N4Os. Molbank 2022, 2022, M1429. https://doi.org/10.3390/M1429
Mambanda A, Kanyora AK, Ongoma P, Gichumbi J, Omondi RO. Chlorido-(η6-p-cymene)-(bis(pyrazol-1-yl)methane-κ2N,N′)Osmium(II) Tetrafluoroborate, C17H22BClF4N4Os. Molbank. 2022; 2022(3):M1429. https://doi.org/10.3390/M1429
Chicago/Turabian StyleMambanda, Allen, Amos K. Kanyora, Peter Ongoma, Joel Gichumbi, and Reinner O. Omondi. 2022. "Chlorido-(η6-p-cymene)-(bis(pyrazol-1-yl)methane-κ2N,N′)Osmium(II) Tetrafluoroborate, C17H22BClF4N4Os" Molbank 2022, no. 3: M1429. https://doi.org/10.3390/M1429
APA StyleMambanda, A., Kanyora, A. K., Ongoma, P., Gichumbi, J., & Omondi, R. O. (2022). Chlorido-(η6-p-cymene)-(bis(pyrazol-1-yl)methane-κ2N,N′)Osmium(II) Tetrafluoroborate, C17H22BClF4N4Os. Molbank, 2022(3), M1429. https://doi.org/10.3390/M1429