Synthesis and Characterization of Two Isostructural POCOP Ni(II) Pincer Complexes Containing Fluorothiophenolate Ligands: [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] and [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}]
Abstract
:1. Introduction
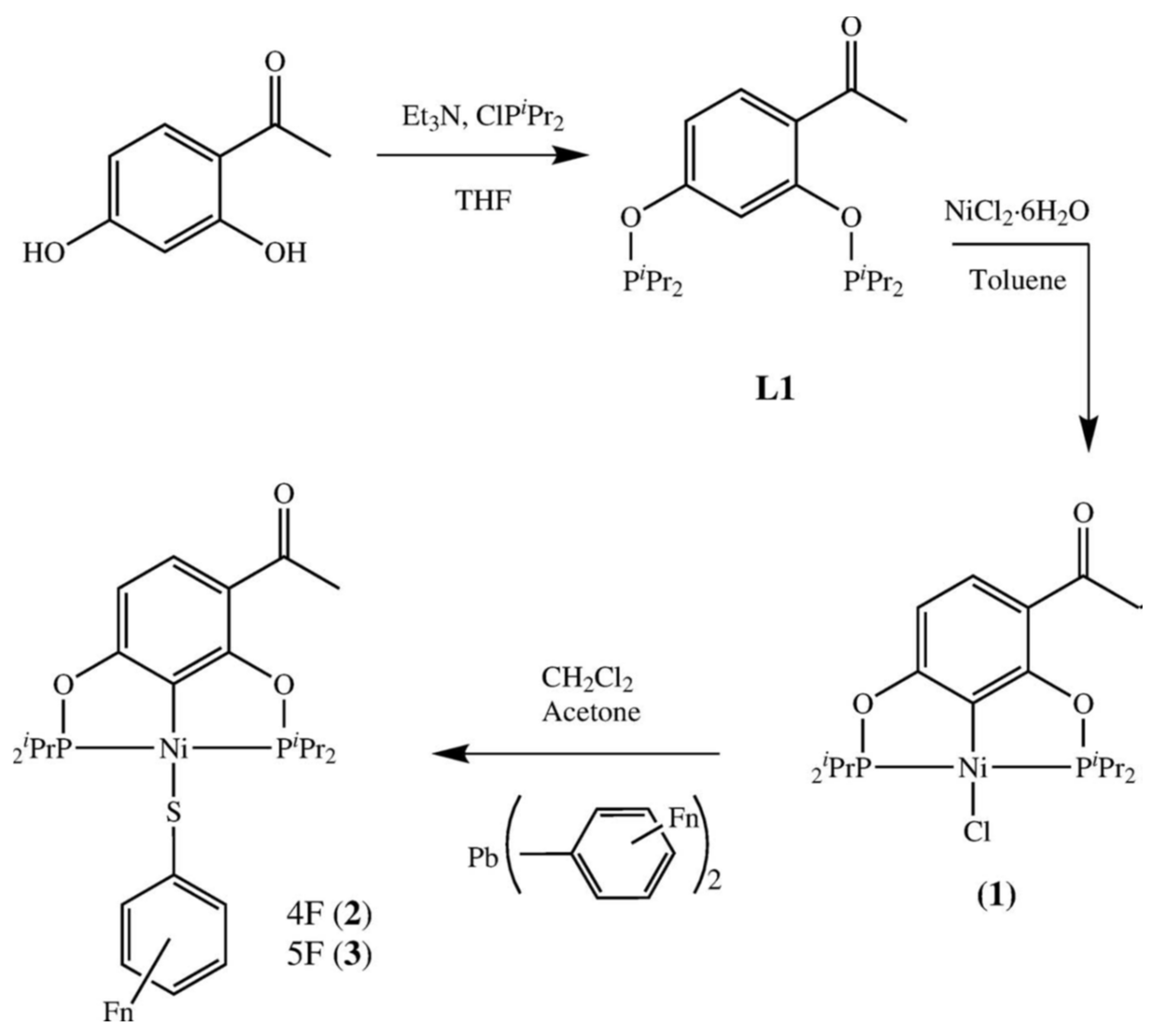
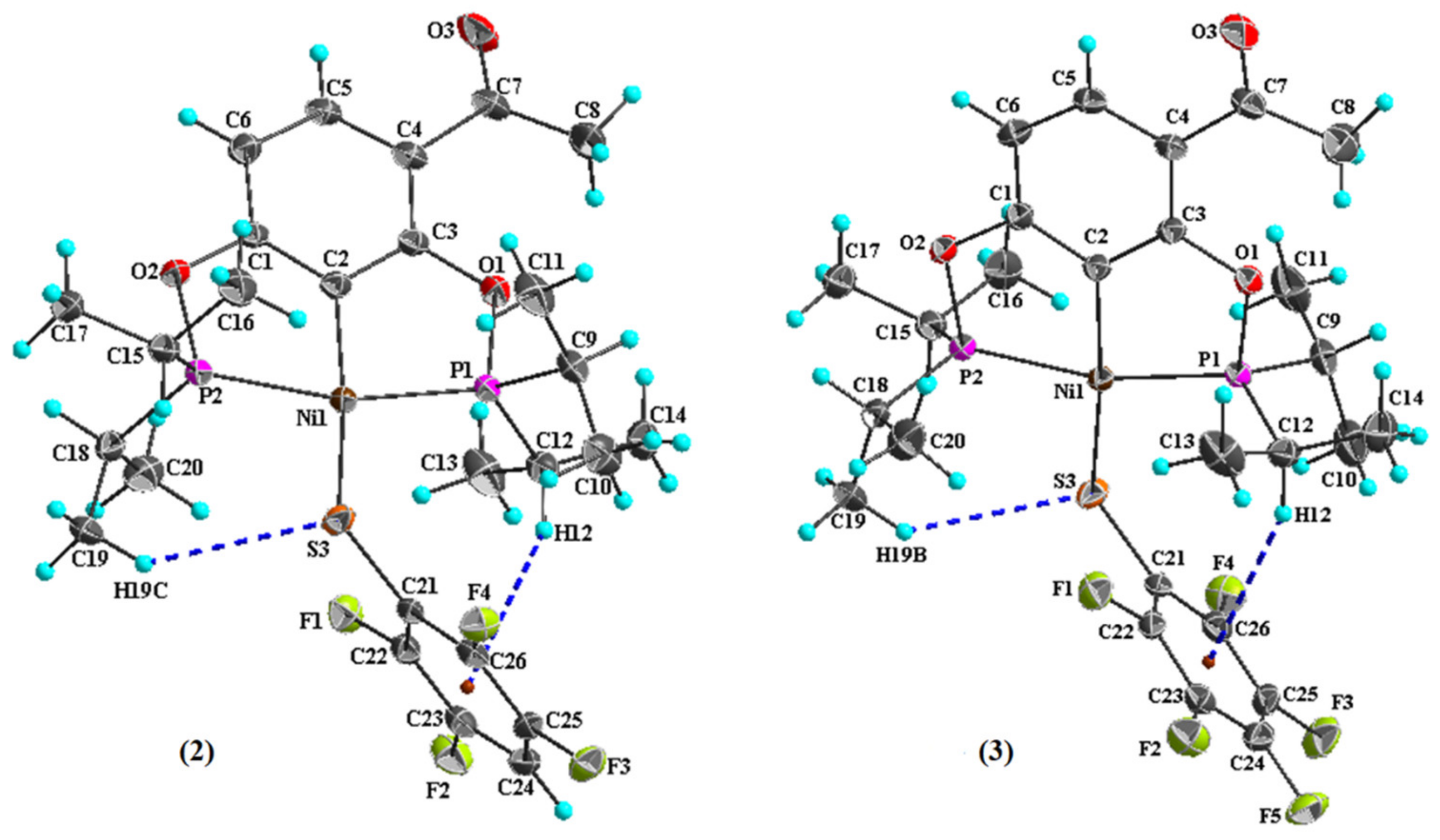
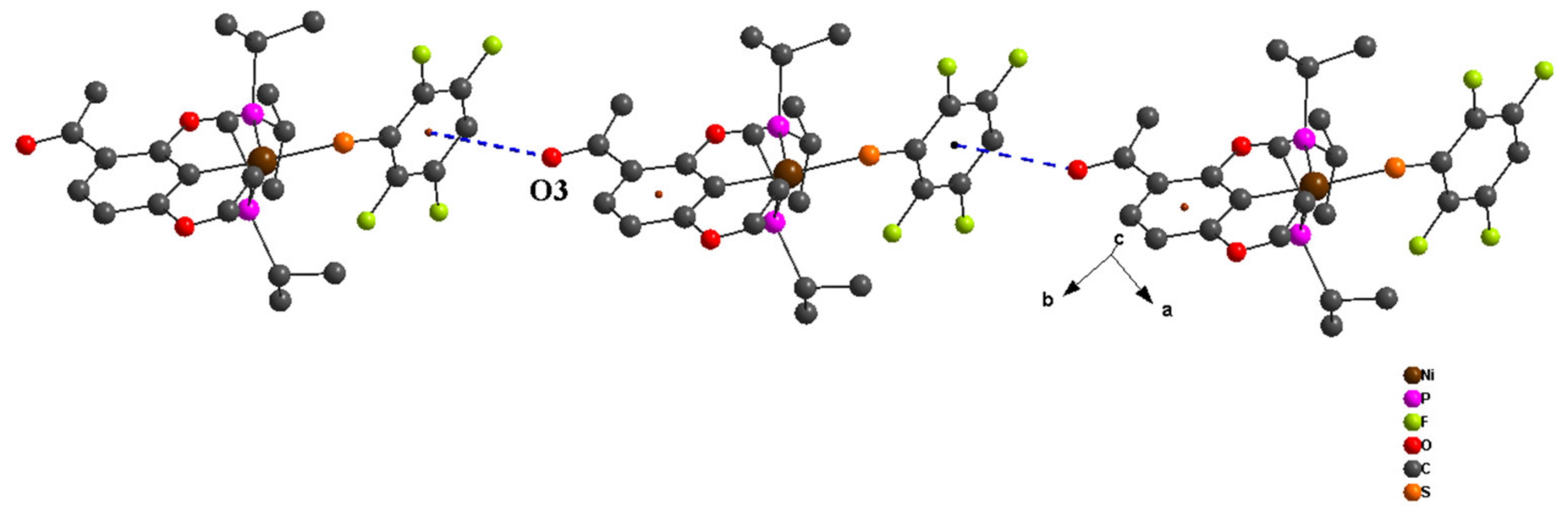
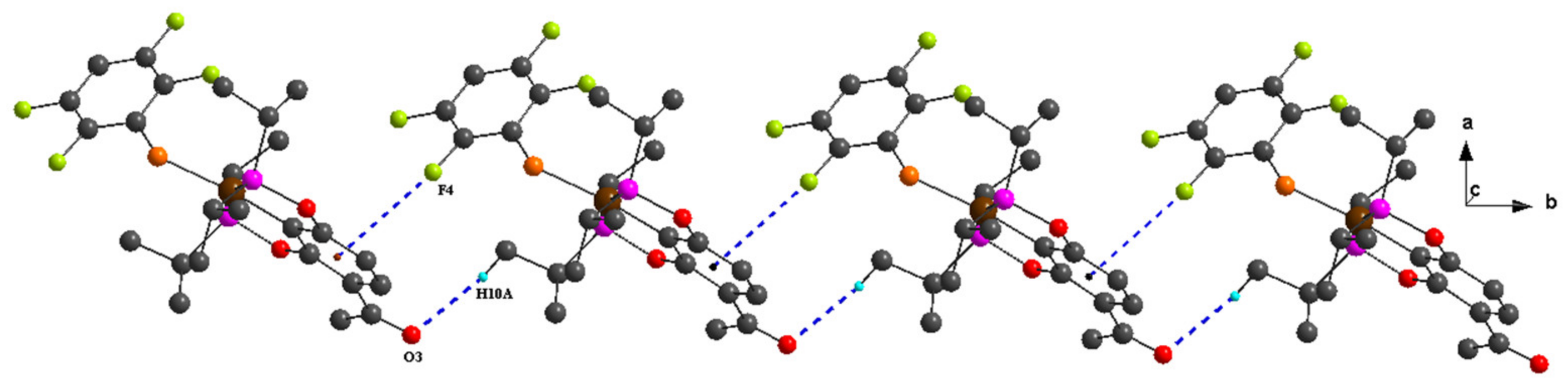
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of the Ligand [C6H2-4-(C2H3O)-1-3-(OPiPr2)2] (L1)
3.3. Synthesis of Complex [NiCl{C6H2-3-(C2H3O)-2-6-(OPiPr2)2}] (1)
3.4. General Procedure for Synthesis of Complexes 2 and 3
3.4.1. [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] (2)
3.4.2. [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] (3)
3.5. Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-Morales, D. The chemistry of PCP pincer phosphinite transition metal complexes. In The Chemistry of the Pincer Compounds, 1st ed.; Morales-Morales, D., Jensen, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 151–180. [Google Scholar]
- Albrecht, M.; Morales-Morales, D. Pincer-Type Iridium Complexes for Organic Transformations. In Iridium Complexes in Organic Synthesis, 1st ed.; Oro, L.A., Claver, C., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 299–323. [Google Scholar]
- Valdés, H.; González-Sebastián, L.; Morales-Morales, D. Aromatic para-functionalized NCN pincer compounds. J. Organomet. Chem. 2017, 845, 229–257. [Google Scholar] [CrossRef]
- Morales-Morales, D. Pincer Complexes. Applications in Catalysis. Rev. Soc. Quim. Mex. 2004, 48, 338–346. [Google Scholar] [CrossRef]
- Asay, M.; Morales-Morales, D. Non-symmetric pincer ligands: Complexes and applications in catalysis. Dalton Trans. 2015, 44, 17432–17447. [Google Scholar] [CrossRef] [PubMed]
- Estudiante-Negrete, F.; Hernández-Ortega, S.; Morales-Morales, D. Ni(II)–POCOP pincer compound [NiCl{C10H5-2,10-(OPPh2)2}] an efficient and robust nickel catalyst for the Suzuki–Miyaura coupling reactions. Inorg. Chim. Acta 2012, 387, 58–63. [Google Scholar] [CrossRef]
- Solano-Prado, M.A.; Estudiante-Negrete, F.; Morales-Morales, D. Group 10 phosphinite POCOP pincer complexes derived from 4-n-dodecylresorcinol: An alternative way to produce non-symmetric pincer compounds. Polyhedron 2010, 29, 592–600. [Google Scholar] [CrossRef]
- Basauri-Molina, M.; Hernández-Ortega, S.; Morales-Morales, D. Microwave-Assisted C–C and C–S Couplings Catalysed by Organometallic Pd-SCS or Coordination Ni-SNS Pincer Complexes. Eur. J. Inorg. Chem. 2014, 4619–4625. [Google Scholar] [CrossRef]
- Suzuki, T. Organic Synthesis Involving Iridium-Catalyzed Oxidation. Chem. Rev. 2011, 111, 1825–1845. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Bond Activation and Catalysis by Ruthenium Pincer Complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef]
- Brown, D.G.; Schauer, P.A.; Borau-Garcia, J.; Fancy, B.R.; Berlinguette, C.P. Stabilization of Ruthenium Sensitizers to TiO2 Surfaces through Cooperative Anchoring Groups. J. Am. Chem. Soc. 2013, 135, 1692–1695. [Google Scholar] [CrossRef]
- Ramírez-Rave, S.; Ramírez-Apan, M.T.; Tlahuext, H.; Morales-Morales, D.; Toscano, R.A.; Grevy, J.M. Non-symmetric CNS-Pt(II) pincer complexes including thioether functionalized iminophosphoranes. Evaluation of their in vitro anticancer activity. J. Organomet. Chem. 2016, 814, 16–24. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. New platinum(II) complexes of CCC-pincer N-heterocyclic carbene ligand: Synthesis, characterization, cytotoxicity and antileishmanial activity. J. Organomet. Chem. 2016, 818, 98–105. [Google Scholar] [CrossRef]
- Choi, J.; MacArthur, A.H.R.; Brookhart, M.; Goldman, A.S. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011, 111, 1761–1779. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef]
- Asay, M.; Morales-Morales, D. Recent Advances on the Chemistry of POCOP–Nickel Pincer Compounds. Top. Organomet. Chem. 2006, 54, 239–268. [Google Scholar]
- Olivos-Suárez, A.I.; Ríos-Moreno, G.; Hernández-Ortega, S.; Toscano, R.A.; García, J.J.; Morales-Morales, D. Reactivity of fluorinated thioether ligands of the type [C6H4Br-2-(CH2SRF)] towards transition metal complexes of the group 10. Inorg. Chim. Acta. 2007, 360, 4133–4141. [Google Scholar] [CrossRef]
- Baldovino-Pantaleón, O.; Hernández-Ortega, S.; Reyes-Martínez, R.; Morales-Morales, D. A second monoclinic polymorph of {2,6-bis[(2,4,5-trifluorophenyl) iminomethyl]pyridine-κ3 N,N′,N″} dichloridonickel(II). Acta Cryst. 2012, E68, m134. [Google Scholar]
- García-Eleno, M.A.; Padilla-Mata, E.; Estudiante-Negrete, F.; Pichal-Cerda, F.; Hernández-Ortega, S.; Toscano, R.A.; Morales-Morales, D. Single step, high yield synthesis of para-hydroxy functionalized POCOP ligands and their Ni(II) pincer derivatives. New J. Chem. 2015, 39, 3361–3365. [Google Scholar] [CrossRef]
- García-Eleno, M.A.; Quezada-Miriel, M.; Reyes-Martínez, R.; Hernández-Ortega, S.; Morales-Morales, D. A comparative study of the packing of two polymorphs of the nickel(II) pincer complex [2,6-bis(di-tert-butylphosphinoyl)-4-(3,5-dinitrobenzoyloxy)phenyl-κ3P,C1,P’]chloridonickel(II). Acta Cryst. 2016, C72, 393–397. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Bruker. APEX2, SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Westrip, S.P.J. publCIF: Software for editing, validating and formatting crystallographic information files. Appl. Cryst. 2010, 43, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? CrystEngComm 2008, 10, 1501–1515. [Google Scholar] [CrossRef]
| (2) CCDC 2142038 | (3) CCDC 2142039 | |
| Crystal data | ||
| Chemical formula | C26H34F4NiO3P2S | C26H33F5NiO3P2S |
| Mr | 623.24 | 641.23 |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n |
| Temperature (K) | 151 | 150 |
| a, b, c (Å) | 8.8138 (11), 10.0715 (13), 31.860 (4) | 8.8159 (3), 10.1816 (3), 31.9637 (12) |
| β (°) | 97.397 (4) | 96.109 (2) |
| V (Å3) | 2804.6 (6) | 2852.77 (17) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.93 | 0.93 |
| Crystal size (mm) | 0.29 × 0.21 × 0.15 | 0.58 × 0.22 × 0.09 |
| Data collection | ||
| Diffractometer | Bruker D8 Venture κ geometry diffractometer 208039-1 | Bruker Smart Apex CCD diffractometer 01-670-03 |
| Absorption correction | Analytical [21] | Analytical [21] |
| Tmin, Tmax | 0.687, 0.820 | 0.616, 0.923 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 39113, 6919, 5212 | 38524, 6810, 5292 |
| Rint | 0.080 | 0.080 |
| (sin θ/λ)max (Å−1) | 0.667 | 0.658 |
| Refinement | ||
| R[F2> 2σ (F2)], wR(F2), S | 0.069, 0.179, 1.11 | 0.048, 0.099, 1.09 |
| No. of reflections | 6919 | 6810 |
| No. of parameters | 343 | 352 |
| H-atom treatment | H-atom parameters constrained w = 1/[σ2(Fo2) + (0.0516P)2 + 13.4197P] where P = (Fo2 + 2Fc2)/3 | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.78, −0.60 | 0.70, −0.34 |
| Ni1–C2 | 1.898 (4) | Ni1–P1 | 2.1662 (12) |
| Ni1–P2 | 2.1524 (13) | Ni1–S3 | 2.2054 (13) |
| C2–Ni1–P2 | 82.12 (14) | C2–Ni1–S3 | 169.35 (14) |
| C2–Ni1–P1 | 81.95 (14) | P2–Ni1–S3 | 90.55 (5) |
| P2–Ni1–P1 | 163.94 (5) | P1–Ni1–S3 | 104.93 (5) |
| Ni1–C2 | 1.902 (3) | Ni1–P1 | 2.1631 (8) |
| Ni1–P2 | 2.1547 (8) | Ni1–S3 | 2.2039 (8) |
| C2–Ni1–P2 | 82.13 (8) | C2–Ni1–S3 | 168.88 (9) |
| C2–Ni1–P1 | 81.98 (9) | P2–Ni1–S3 | 90.44 (3) |
| P2–Ni1–P1 | 164.09 (3) | P1–Ni1–S3 | 105.16 (3) |
| D–H ··A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
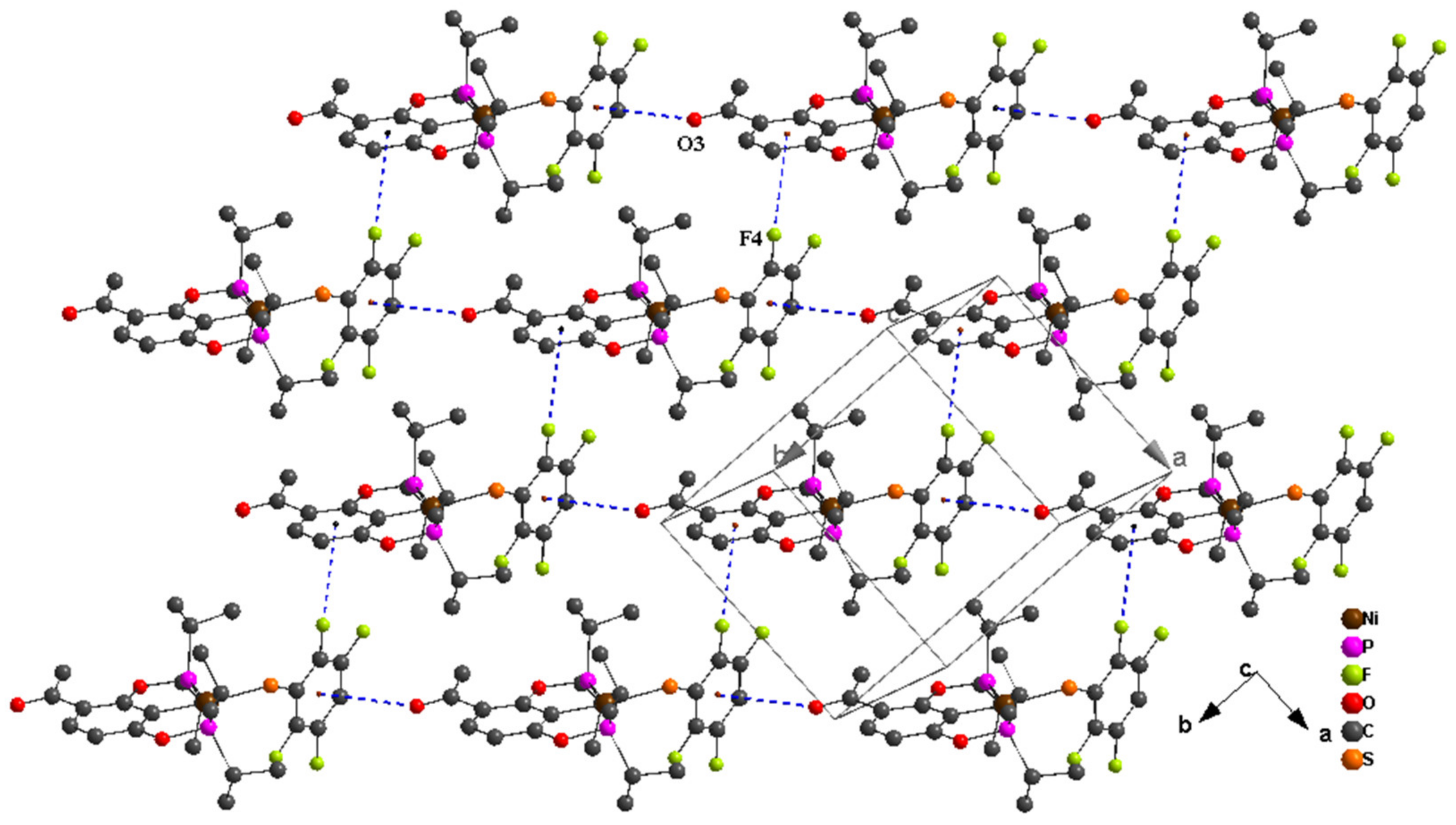
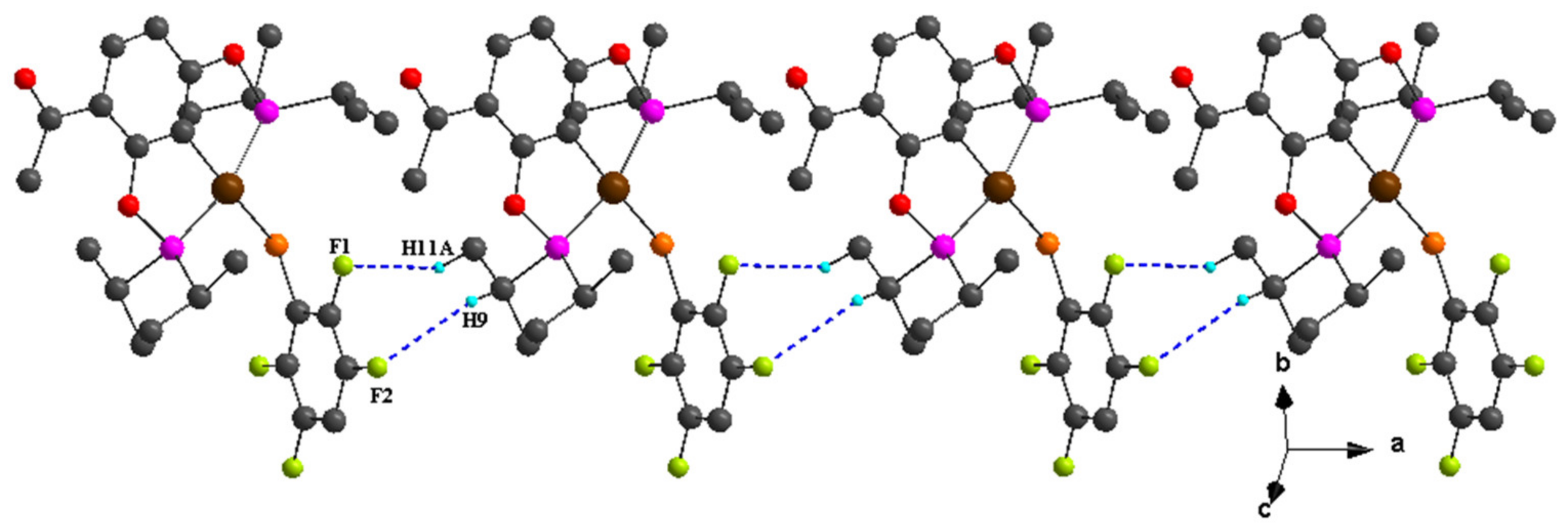
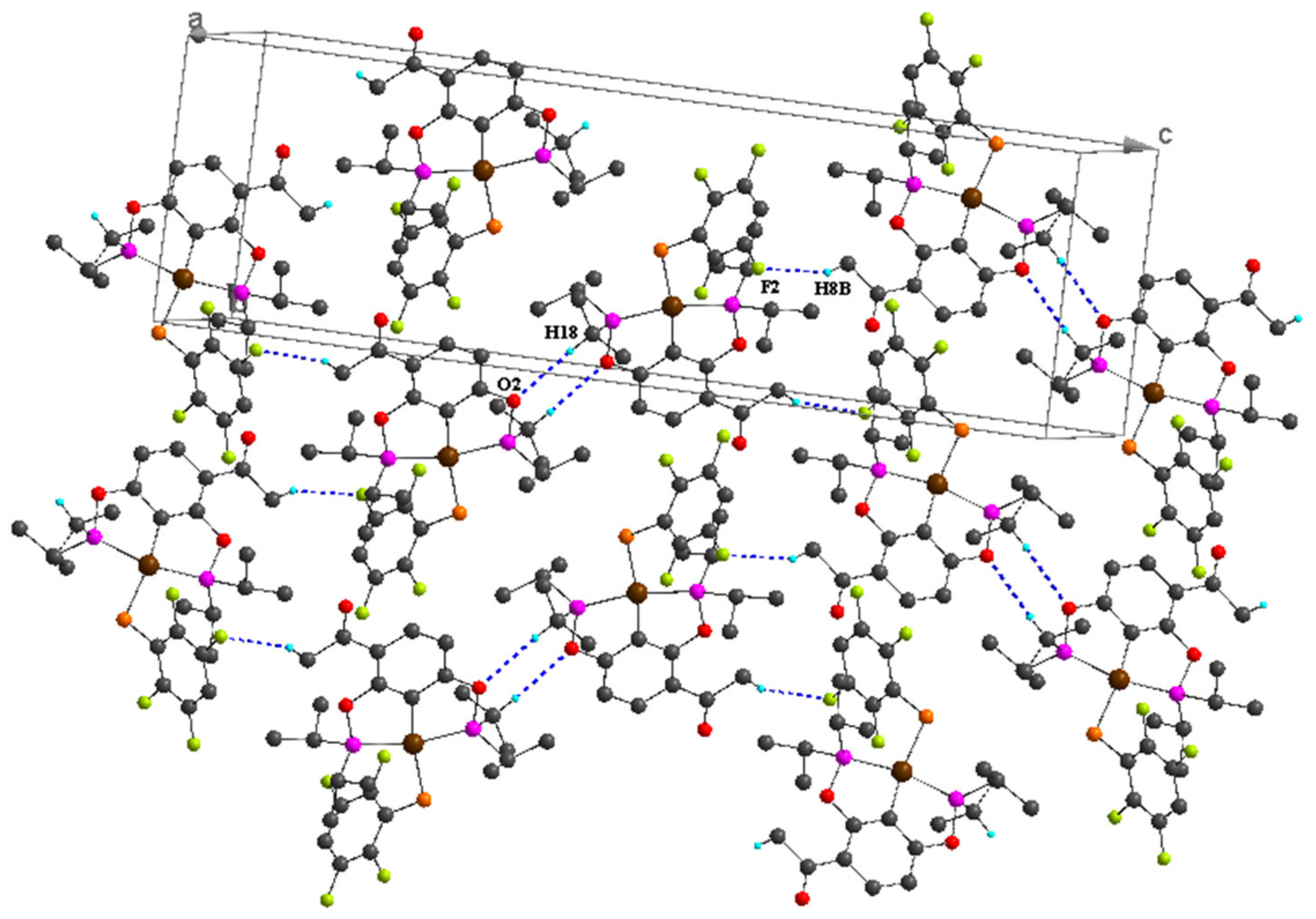
| C8–H8B···F2 i | 0.98 | 2.50 | 3.352 (6) | 145 |
| C10–H10A···O3 ii | 0.98 | 2.47 | 3.418 (7) | 163 |
| C10–H10C···F4 | 0.98 | 2.53 | 3.373 (7) | 144 |
| C13–H13C···F1 | 0.98 | 2.50 | 3.307 (7) | 140 |
| C18–H18···O2 iii | 1.00 | 2.64 | 3.615 (6) | 164 |
| C19–H19C···S3 | 0.98 | 2.95 | 3.740 (5) | 138 |
| C20–H20B···F1 | 0.98 | 2.59 | 3.572 (7) | 176 |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| C8–H8C···F2 i | 0.98 | 2.49 | 3.312 (4) | 141 |
| C9–H9···F2 ii | 1.00 | 2.62 | 3.476 (3) | 144 |
| C10–H10A···O3 iii | 0.98 | 2.52 | 3.458 (4) | 160 |
| C10–H10B···F4 | 0.98 | 2.52 | 3.394 (4) | 149 |
| C13–H13B···F1 | 0.98 | 2.59 | 3.325 (4) | 132 |
| C19–H19B···S3 | 0.98 | 2.98 | 3.745 (3) | 136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Espinosa, E.G.; Ortiz-Pastrana, N.; Gómez-Benítez, V.; Reyes-Martínez, R.; Piñón-Castillo, H.A.; Manjarrez-Nevárez, L.A.; German-Acacio, J.M.; Morales-Morales, D. Synthesis and Characterization of Two Isostructural POCOP Ni(II) Pincer Complexes Containing Fluorothiophenolate Ligands: [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] and [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}]. Molbank 2022, 2022, M1359. https://doi.org/10.3390/M1359
Morales-Espinosa EG, Ortiz-Pastrana N, Gómez-Benítez V, Reyes-Martínez R, Piñón-Castillo HA, Manjarrez-Nevárez LA, German-Acacio JM, Morales-Morales D. Synthesis and Characterization of Two Isostructural POCOP Ni(II) Pincer Complexes Containing Fluorothiophenolate Ligands: [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] and [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}]. Molbank. 2022; 2022(2):M1359. https://doi.org/10.3390/M1359
Chicago/Turabian StyleMorales-Espinosa, Eric G., Naytze Ortiz-Pastrana, Valente Gómez-Benítez, Reyna Reyes-Martínez, Hilda Amelia Piñón-Castillo, Laura A. Manjarrez-Nevárez, Juan M. German-Acacio, and David Morales-Morales. 2022. "Synthesis and Characterization of Two Isostructural POCOP Ni(II) Pincer Complexes Containing Fluorothiophenolate Ligands: [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] and [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}]" Molbank 2022, no. 2: M1359. https://doi.org/10.3390/M1359
APA StyleMorales-Espinosa, E. G., Ortiz-Pastrana, N., Gómez-Benítez, V., Reyes-Martínez, R., Piñón-Castillo, H. A., Manjarrez-Nevárez, L. A., German-Acacio, J. M., & Morales-Morales, D. (2022). Synthesis and Characterization of Two Isostructural POCOP Ni(II) Pincer Complexes Containing Fluorothiophenolate Ligands: [Ni(SC6F4-4-H){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}] and [Ni(SC6F5){C6H2-3-(C2H3O)-2,6-(OPiPr2)2}]. Molbank, 2022(2), M1359. https://doi.org/10.3390/M1359








