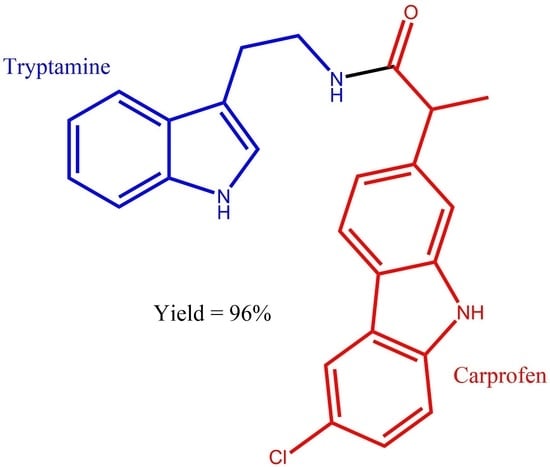
N-(2-(1H-indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide
Abstract
:1. Introduction
2. Results
3. Materials and Methods
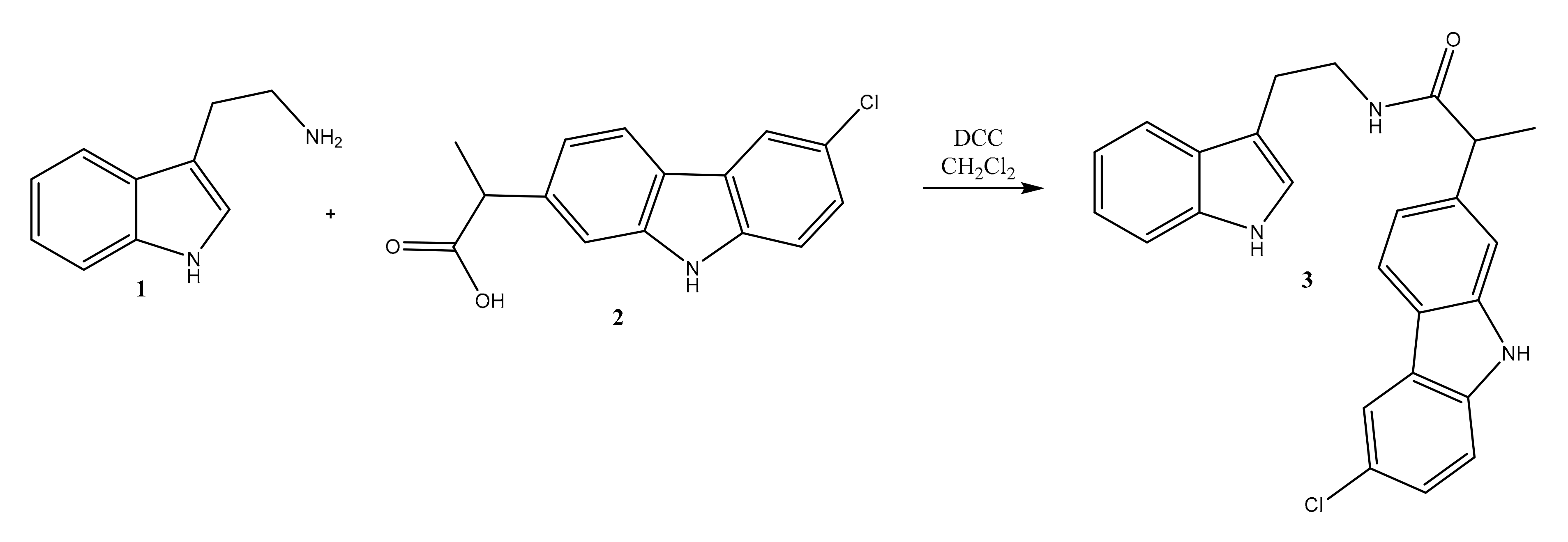
Synthesis of N-(2-(1H-Indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide 3
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Melnikova, I. Future of COX2 inhibitors. Nat. Rev. Drug Discov. 2005, 4, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Novel non-steroidal anti-inflammatory drugs. Bailliere’s Clin. Rheumatol. 1988, 2, 485–511. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medicinal Products, EMEA/MRL/042/95-FINAL/Committee for Veterinary Medicinal Products/Carprofen. Available online: https://www.ema.europa.eu/en/documents/mrl-report/carprofen-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 20 October 2020).
- Kousara, S.; Anjuma, S.; Jaleela, F.; Khana, J.; Naseema, S. Biomedical Significance of Tryptamine: A Review. J. Pharmacovigil. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Lhiaubet-Vallet, V.; Boscá, F.; Miranda, M. Stereodifferentiating drug-biomolecule interactions in the triplet excited state: Studies on supramolecular carprofen/protein systems and on carprofen-tryptophan model. J. Phys. Chem. B 2007, 111, 423–431. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D. N-(2-(1H-indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide. Molbank 2020, 2020, M1171. https://doi.org/10.3390/M1171
Manolov S, Ivanov I, Bojilov D. N-(2-(1H-indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide. Molbank. 2020; 2020(4):M1171. https://doi.org/10.3390/M1171
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, and Dimitar Bojilov. 2020. "N-(2-(1H-indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide" Molbank 2020, no. 4: M1171. https://doi.org/10.3390/M1171
APA StyleManolov, S., Ivanov, I., & Bojilov, D. (2020). N-(2-(1H-indol-3-yl)ethyl)-2-(6-chloro-9H-carbazol-2-yl)propanamide. Molbank, 2020(4), M1171. https://doi.org/10.3390/M1171