Abstract
A new pyranocoumarin, namely 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut- 3″en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one 1, was isolated from the stem barkof Calophyllum pesudomole. The structure of compound 1 was elucidated based on its ultaraviolet (UV); infrared (IR); high resolution electro spray ionization mass spectrometry (HRESIMS); 1D and 2D nuclear magnetic resonance (NMR) spectral data.
1. Introduction
The genus Calophyllum belongs to the Clusiaceae family which comprises about 180 species found mainly in Southeast Asia. This plant isendemic to Kalimantan Island, Indonesia. This genus has been shown to possess a number of secondary metabolites such as xanthones [1,2,3], coumarins [4,5], and chromanone acids [6,7]. Many of these compounds have shown a wide range of biological and pharmacological properties such as anti-HIV [8,9], anticancer [10,11], and antimalarial properties [12].
In continuation of our phytochemical investigation on bioactive xanthone, we wish to report the isolation and structural elucidation of a new pyranoxanthone, 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one (Figure 1) from the stem bark of Calophyllum pseudomole. The cytotoxicity against murine leukemia P-388 and antiplasmodial activity against Plasmodium falciparum of 1 are also briefly described.
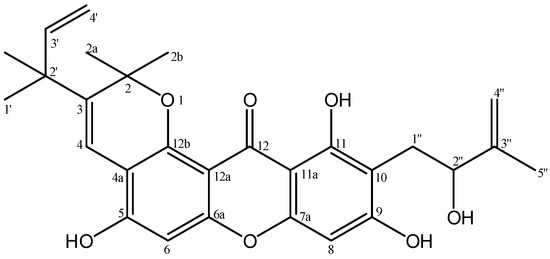
Figure 1.
Structures of 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2- dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one.
2. Result and Discussion
5,9,11-Trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-onewas isolated as a yellow solid, and its UV absorption maxima at λmax 245, 268, and 320 nm which is characteristic of xanthone chromophore [2]. Based on HRESIMS (see data supplementary Figure S1), compound 1 showed deprotonated molecule ion [M− H]− at m/z 477.1917corresponding to the molecular formula C28H30O7. The IR spectrum (data supplementary Figure S2) showed absorption for hydroxyl (3423 cm−1), conjugated carbonyl (1645 cm−1), and aromatic (1577 and 1460 cm−1) groups [13]. The 1H-NMR spectrum (Table 1, data supplementary Figure S3) of 1 showed the presence of a singlet of chelated hydroxyl at δH13.82 (11-OH), a singlet of hydroxyl at δH 6.14 (5-OH), and two singlets of the isolated aromatic proton at δH 6.81 (H-6) and δH 6.39 (H-8) which is typical for a xanthone with six substituents [1]. The 1H-NMR spectrum also revealed the presence of a substituent of a monosubstitued 2,2-dimethylpyrano, 2-methylbut-3-en-2-yl, and 2-hydroxy-3-methylbut-3-en-1-yl group. The presence of a monosubstitued 2,2-dimethylpyrano was determined from resonances of vinyl at δH 8.13 (s, 1H), and two signals of methyl at δH 1.54 (s, 3H) and 1.52 (s, 3H). The 1H-NMR spectrum of 2-methylbut-3-en-2-yl showed the presence of a methyl at δH 1.43 (s, 6H), a vinyl at δH 5.98 (dd, 1H), and a methylene at δH 5.13 (d, 1H) and δH 5.08 (d, 1H). The 1H-NMR spectrum of 2-methylbut-3-en-2-yl also showed the presence of a methyl at δH 1.87 (s, 3H), two proton signals of methylene at δH 3.16 (dd, 1H) and 2.93 (dd, 1H), a methyne at δH 4.43 (d, 1H), and two proton signals of vinilyc methylene at δH 4.97 (s, 1H) and 4.86 (s, 1H).

Table 1.
NMR spectroscopic data of 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)- 2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one in CDCl3.
The 13C-NMR spectrum (Table 1, Figure S4 data supplementary) of 1 showed 28 carbon signals and their assignments were determined by heteronuclear multiple quantum coherence (HMQC) and heteronuclear multiple bond correlation (HMBC) spectra. The placement of hydroxyl, a monosubstitued 2,2-dimethylpyrano, 2-methylbut-3-en-2-yl, and 2-hydroxy-3-methylbut-3-en-1-yl group was confirmed by HMQC and HMBC spectra(Table 1). Based on the HMBC spectrum, a chelated hydroxyl signal at δH 13.82 (11-OH) was correlated with two quaternary carbons [δC 107.4 (C-10); 103.4 (C-11a)], and an oxyaryl carbon δC 161.1 (C-11) indicated that C-10 has a substitutent. Two proton signals of methylene (H-1″) of the 2-hydroxy-3-methylbut-3-en-1-yl group at δH3.18 and δH 2.95 showed long-range correlations with two oxyaryl carbons [δC 161.1; 163.2 (C-9)], two quaternary carbons [δC 107.4; 146.7 (C-3″)], and a methine carbon of alcohol [δC 77.5 (C-2″)]. The correlations showed that the 2-hydroxy -3-methylbut-3-en-1-ylgroup attached at C-10. The position of 2-hydroxy-3-methylbut-3-en-1-yl group at C-10 is reinforced by the correlation of the isolated aromatic proton signals at δH6.39 (H-8) with two oxyaryl carbons [δC 155.8 (C-7a); 163.2 (C-9)], and two quaternary carbons [δC107.4 (C-10); 103.4(C-11a)]. Furthermore, the proton signal of isolated aromatic at δH 6.81 (H-6) correlates with two oxyaril carbons [δC 153.4 (C-5), 150.6 (C-6a)] and a quaternary carbon signal (δC 108.5, C-4a), which showed that 2,2-dimethylpyrano was fused at C-4a and C-12b and thehydroxyl group attached at C-5 from the xanthone skeleton. The position of the hydroxyl group at C-5 is reinforced by the correlation of the hydroxyl proton signal at δH 6.15 (5-OH) with an oxyaryl carbon [δC 153.4 (C-5)], and a methine carbon [δC 101.8 (C-6)]. The methine signal of the vinyl group at δH 8.13 (H-4) of a monosubstitued 2,2-dimethylpyrano group showed long-range correlations with four quarternary carbons [δC 80.6 (C-2), 135.7 (C-3), 108.5 (C-4a), 42.1 (C-2′)] which showed thata 2-methyl but-3-en-2-yl group attached at C-3. The HMBC correlation of compound 1 can seen on Figure 2. Therefore, compound 1 was identified as 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl) -2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one and a novel compound.
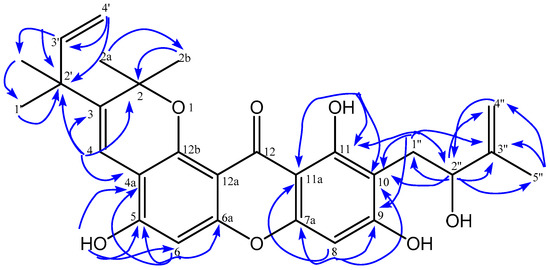
Figure 2.
Selected HMBC correlation for 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)- 2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xathen-12-one.
The cytotoxic activityof compound 1 against murine leukemia P-388 cells, as determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay, showed IC50 values of 4.10 μg/mL andwas categorized as moderate activity. The antiplasmodial activity of 1 against P. falciparum showed IC50 values of 2.88 μg/mL and was categorized as moderate activity.
3. Experiment Section
3.1. General
NMR spectra were recorded on a JEOL 400 ECA spectrophotometer (Tokyo, Japan) in CDCl3 at 400 (1H) and 100 (13C) MHz using tetramethyl silane (TMS) as the internal standard. The mass spectra were recorded using a Waters LCT Premier XE (Santa Clara, CA, USA). The UV spectra were measured with a Shimadzu series 1800 spectrophotometer (Kyoto, Japan). The IR spectra were recorded with Perkin–Elmer spectrum-100 FT-IR (Waltham, MA, USA). Column chromatography and planar radial chromatography were carried out using silica gel 60 G 1.07734.1000 and Si gel 60 PF254 1. 07749.1000 (Merck, Darmstadt, Germany).
3.2. Plant Material
The stem bark of C. Pseudomolewas collected in Sungai Mendawak, anak Sungai Kapuas, District Kubu Raya, Kalimantan, Indonesia on April 2015. The specimen was identified at the Herbarium Bogoriense, Center of Biological Research and Development, National Institute of Science, Bogor, Indonesia.
3.3. Extraction and Isolation
The dried stem bark of C. pseudomole (3.0 kg) was macerated in MeOH twice for 2 days each time. After evaporation of the solvent in a rotary evaporator, 260 g of pale brown semi-solid was obtained. Further, the MeOH extract was partitioned first with n-hexane (1:1 v/v); water was added (10% v/v) in the second step to increase the polarity; then the MeOH extract was partitioned with EtOAc (1:1 v/v). The EtOAc extract (35 g)was subjected to column chromatography over silica gel and eluted with n-hexane-ethyl acetate (from 9:1 to 1:1) to give fractions A–C. Fraction C was then subjected further to column chromatography and eluted with n-hexane-EtOAc (from 9:1 to 1:1) to produce three subfractions C1–C3. Subfraction C2 was purified by planar radial chromatography usingn-hexane-CHCl3 (from 3:7 to 7:3), CHCl3 and CHCl3-EtOAc 9:1 to yield compound 5,9,11-trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one (18 mg).
3.4. Cytotoxic Assay
The cytotoxic properties of 1 against murine leukemia P-388 cells were evaluated according to the MTT method as previosly described [14,15,16]. Artonin E was used as the positive control.
3.5. Antiplasmodial Assay
The antiplasmodial properties of 1 against P. falciparum were evaluated according to the Trager–Jensen method as previosly described [17,18]. Chloroquine was used as the positive control.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2017/4/M961/s1, IR, HRESIMS, 1D and 2D NMR spectra are reported in the supplementary materials as Figures S1–S6.
Acknowledgments
This research was supported by Universitas Airlangga, Ministry of Research, Technology and Higher Education, Republic of Indonesia (Penelitian Hibah Kompetensi, Universitas Airlangga, 2017).
Author Contributions
Mulyadi Tanjung designed the whole experiment of bioactivity and wrote the manuscript. Tjitjik Srie Tjahjandarie researched data, analyzed the NMR and HRESIMS spectra and contributed to the manuscript, Ratih Dewi Saputri designed the whole experiment. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. 5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one from the stem bark of Calophyllum pseudomole. Molbank 2016, M906. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Saputri, R.D.; Tanjung, M. 5,9,11-Trihydroxy-2,2-dimethyl-3-(2-methylbut-3-en-2-yl) pyrano[2,3-a]xanthen-12(2H)-one from the stem bark of Calophyllum tetrapterum Miq. Molbank 2017, M936. [Google Scholar] [CrossRef]
- Wei, D.J.; Mei, W.L.; Zhong, H.M.; Zeng, Y.B.; Wu, X.D.; Dai, H.F. A new prenylated xanthone from the branches of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.B.; Ee, G.C.L.; Malek, E.A.; Teh, S.S.; See, I. A new coumarin from Calophyllum hosei. Nat. Prod. Res. 2014, 28, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.P.; Kulkarni, S.R.; Phalgune, U.D.; Puranik, V.G. New dipyranocoumarin from the leaves of Calophyllum apetalum Willd. Nat. Prod. Res. 2013, 27, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.D.; Hansen, P.E.; Duus, F.; Pham, H.D.; Nguyen, L.D. A new chromanone acid from the bark of Calophyllum dryobalanoides. Phytochem. Lett. 2012, 5, 287–291. [Google Scholar] [CrossRef]
- Lim, C.K.; Subramaniam, H.; Say, Y.H.; Jong, V.Y.M.; Khaledi, H.; Chee, C.F. A new chromanone acid from the stem bark of Calophyllum teysmannii. Nat. Prod. Res. 2015, 29, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Covington, C.D.; Fuller, R.W.; Bokesch, H.R.; Young, S.; Cardellina, J.H.; Kadushin, M.R.; Soejarto, D.D.; Stevens, P.F.; Cragg, G.M. Pyranocoumarins from tropical species of the genus Calophyllum: Chemotaxonomic study of extracts in the national cancer institute collection. J. Nat. Prod. 1998, 61, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.H.; Basualdo, M.C.; Abe, F.; Estrada, M.J.; Soler, C.; Chilpa, R.R. HIV-1 inhibitory compounds from Calophyllum brasiliense leaves. Biol. Pharm. Bull. 2004, 27, 1471–1475. [Google Scholar] [CrossRef]
- Xiao, Q.; Zeng, Y-B.; Mei, W.L.; Zhao, Y-X.; Deng, Y-Y.; Dai, H-F. Cytotoxic prenylated xanthones from Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2008, 10, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.H.; Ee, G.C.L.; Teh, S.S.; Sukari, M.A. Calophyllum inophyllum and Calophyllum soulattri source of anti-proliferative xanthones and their structure-activity relationships. Nat. Prod. Res. 2013, 27, 98–101. [Google Scholar]
- Hay, A.; Helesbeux, J.; Duval, O.; Labaied, M.; Grellier, P.; Richomme, P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004, 75, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepinderivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
- Tanjung, M.; Hakim, E.H.; Syah, Y.M. Prenylated dihydrostilbenes from Macaranga rubiginosa. Chem. Nat. Compd. 2017, 53, 215–218. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Trop. Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Hakim, E.H.; Elfahmi; Latip, J.; Syah, Y.M. Dihydroflavonol and flavonol derivatives from Macaranga recurvata. Nat. Prod. Commun. 2012, 10, 1309–1310. [Google Scholar]
- Tanjung, M.; Saputri, R.D.; Wahjoedi, R.A.; Tjahjandarie, T.S. 4-Methoxy-3-(3-methylbut-2-en-1-yl)-7-(3-methylbut-2-en-1-yl)oxy)quinolin-2(1H)-one from Melicope moluccana T.G. Hartley. Molbank 2017, M939. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Pudjiastuti, P.; Saputri, R.D.; Tanjung, M. Antimalaria and antioxidant activity of phenolic compounds isolated from Erythrina crysta-galli L. J. Chem. Pharm. Res. 2014, 6, 786–790. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).