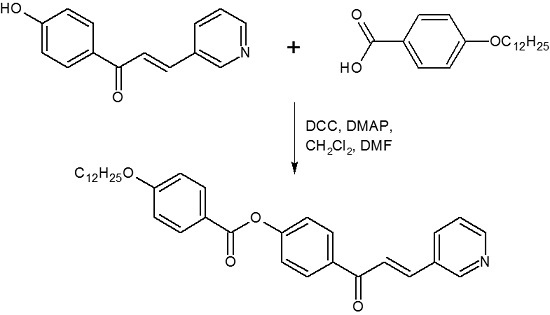
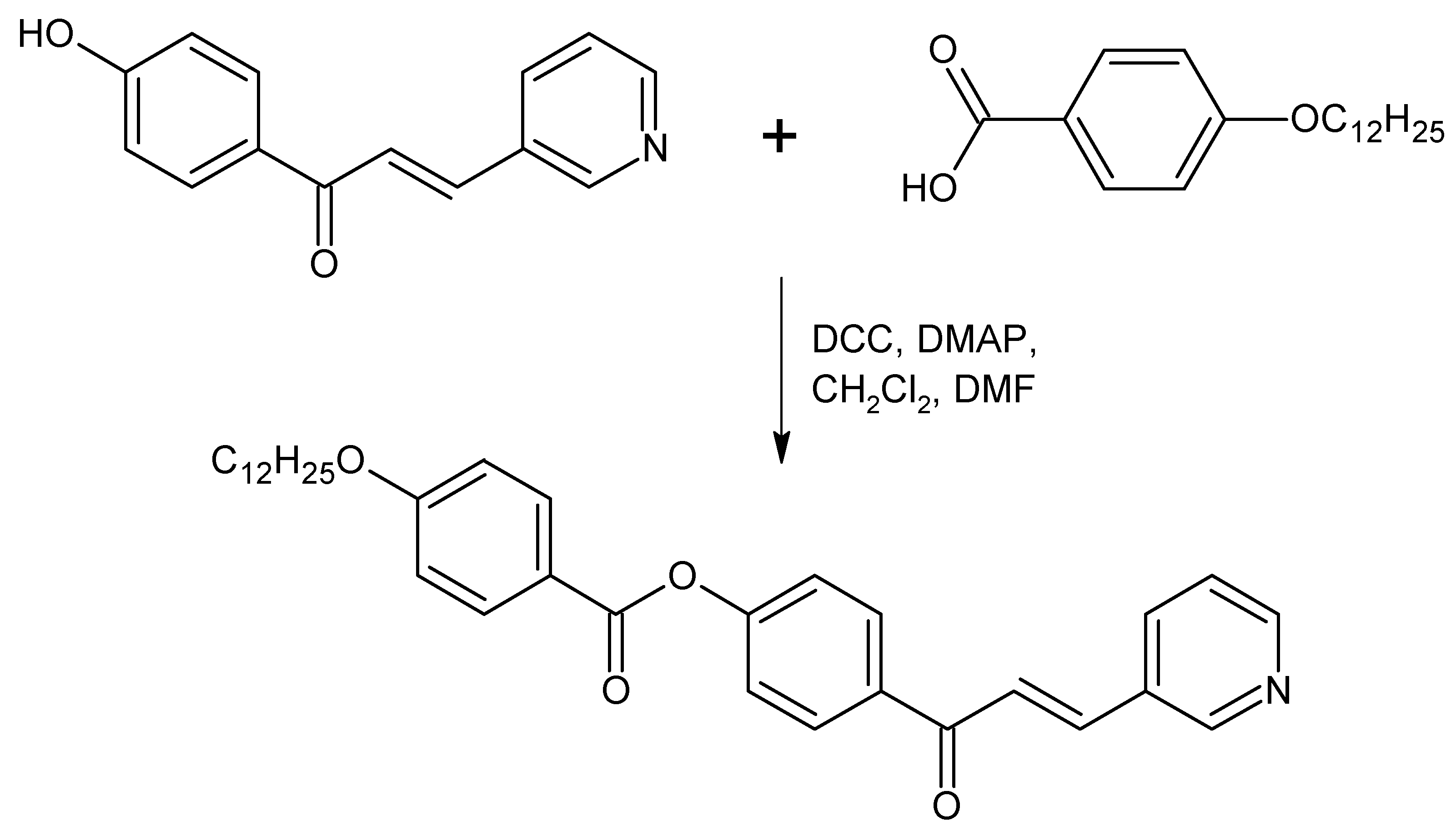
4-[3-(Pyridin-3-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoate
Abstract
:Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seed, A. Synthesis of self-organizing mesogenic materials containing a sulfur-based five-membered heterocyclic core. Chem. Soc. Rev. 2007, 36, 2046–2069. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.L.; Saavedra, C.G.; Hildago, P.I.; Elgueta, E.Y. Novel chiral liquid crystals based on amides and azo compounds derived from 2-amino-1,3,4-thiadiazoles: synthesis and mesomorphic properties. Liq. Cryst. 2008, 35, 55–64. [Google Scholar] [CrossRef]
- Ha, S.T.; Koh, T.M.; Lin, H.C.; Yeap, G.Y.; Win, Y.F.; Ong, S.T.; Sivasothy, Y.; Ong, L.K. Heterocyclic benzothiazole-based liquid crystals: synthesis and mesomorphic. Liq. Cryst. 2009, 36, 917–925. [Google Scholar] [CrossRef]
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Lee, S.L.; Win, Y.F.; Ong, S.T. Mesogenic Schiff base esters with benzothiazole core: synthesis and phase transition studies. Phase Trans. 2010, 83, 195–204. [Google Scholar] [CrossRef]
- Koh, T.M.; Ha, S.T.; Yeap, G.Y.; Lin, H.C. New mesomorphic benzothiazoyl derivatives: synthesis and characterization. Chin. Chem. Lett. 2013, 24, 926–828. [Google Scholar] [CrossRef]
- Song, X.; Li, J.; Zhang, S. Supramolecular liquid crystals induced by intermolecular hydrogen bonding between benzoic acid and 4-(alkoxyphenylazo) pyridines. Liq. Cryst. 2003, 30, 331–335. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Yeap, G.Y. Synthesis and phase transition behaviors of a homologous series of pyridine symmetrical dimmers. Asian J. Chem. 2013, 25, 6151–6155. [Google Scholar]
- Ha, S.T.; Low, Y.W. Synthesis and phase transition behaviors of new chalcone derivatives. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.-T.; Lau, Y.-J. 4-[3-(Pyridin-3-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoate. Molbank 2015, 2015, M879. https://doi.org/10.3390/M879
Ha S-T, Lau Y-J. 4-[3-(Pyridin-3-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoate. Molbank. 2015; 2015(4):M879. https://doi.org/10.3390/M879
Chicago/Turabian StyleHa, Sie-Tiong, and Yi-Jun Lau. 2015. "4-[3-(Pyridin-3-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoate" Molbank 2015, no. 4: M879. https://doi.org/10.3390/M879
APA StyleHa, S.-T., & Lau, Y.-J. (2015). 4-[3-(Pyridin-3-yl)prop-2-enoyl]phenyl 4-dodecyloxybenzoate. Molbank, 2015(4), M879. https://doi.org/10.3390/M879