1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one
Abstract
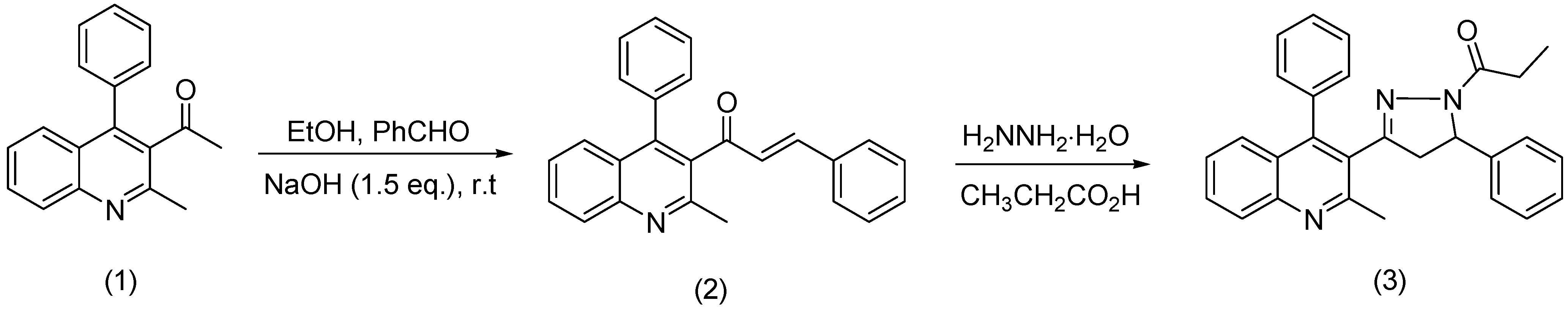
:
Experimental Section
Synthesis of 1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Author Contributions
Conflicts of Interest
References
- Morimoto, Y.; Matsuda, F.; Shirahama, H. Total Synthesis of (±)-Virantmycin and Determination of Its Stereochemistry. Synlett 1991, 1991, 202–203. [Google Scholar] [CrossRef]
- Markees, D.G.; Dewey, V.C.; Kidder, G.W. Antiprotozoal 4-aryloxy-2-aminoquinolines and related compounds. J. Med. Chem. 1970, 13, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.S.; Hardstone, J.D.; Palmer, J.M. 2,4-Diamino-6,7-dimethoxyquinoline derivatives as .alpha.1-adrenoceptor antagonists and antihypertensive agents. J. Med. Chem. 1988, 31, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.P.; Sheets, K.R.; Zilberstein, A.A. New Series of PDGF Receptor Tyrosine Kinase Inhibitors: 3-Substituted Quinoline Derivatives. J. Med. Chem. 1994, 37, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Darío Vélez, I.; Upegui, Y.; Muňoz, J.A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo[3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Darío Vélez, I.; Upegui, Y.; Muňoz, J.A.; Nogueras, M.; et al. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M.; Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Sarveswari, S.; Vijayakumar, V. An Efficient Microwave Assisted Eco-friendly Synthesis of 6-Chloro-3-(3-arylacryloyl)-2-methyl-4-phenylquinolines and their Conversion to 6-Chloro-3-(1-phenyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl)-2-methyl-4-phenylquinolines. J. Chin. Chem. Soc. 2012, 59, 66–71. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Rao, A.L.; Prasoona, L.; Murali, K.; Kumar, P.R. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxynaphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Negi, J.S.; Pant, G.J.N.; Rawat, M.S.M.; Budakoti, A. Synthesis and Characterization of a Novel 2-Pyrazoline. Molbank 2009, 2009, M614. [Google Scholar] [CrossRef]
- Loh, W.-S.; Fun, H.-K.; Prasath, R.; Sarveswari, S.; Vijayakumar, V. (E)-1-(2-Methyl-4-phenylquinolin-3-yl)-3-phenylprop-2-en-1-one. Acta Crystallogr. 2011, E67, o764–o765. [Google Scholar] [CrossRef] [PubMed]
- Kotra, V.; Ganapaty, S.; Adapa, S.R. Synthesis of new series of quinolinyl chalcones as anticancer and anti-inflammatory agents. Indian J. Chem. Sec. B 2010, 49, 1109–1116. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedjadja, A.; Kolli, E.; Bouraiou, A.; Merdes, R. 1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one. Molbank 2015, 2015, M863. https://doi.org/10.3390/M863
Kedjadja A, Kolli E, Bouraiou A, Merdes R. 1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one. Molbank. 2015; 2015(2):M863. https://doi.org/10.3390/M863
Chicago/Turabian StyleKedjadja, Allaoua, Elhadj Kolli, Abdelmalek Bouraiou, and Rachid Merdes. 2015. "1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one" Molbank 2015, no. 2: M863. https://doi.org/10.3390/M863
APA StyleKedjadja, A., Kolli, E., Bouraiou, A., & Merdes, R. (2015). 1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one. Molbank, 2015(2), M863. https://doi.org/10.3390/M863




