Abstract
A new tribenzamidomethyl hydrazine was synthesized and its IR, 1H NMR, 13C NMR and MS spectroscopic data are presented.
Many hydrazine derivatives are known to exhibit significant biological activity and several compounds with hydrazine moiety were shown to be effective for treatment of tuberculosis, Parkinson’s disease and hypertension [1]. There is evidence that some hydrazines displaying neuroprotective properties can be used as antidepressant drugs [2], while for some trisubstituted hydrazines antimicrobial effect was observed [3]. The hydrazine moiety has been used for modification of peptides as well. For example, novel building blocks for peptide modification have been designed and synthesized to obtain β-sheet tripeptide mimics that comprise the azapeptide modification at one end [4]. Azapeptides, hydrazine-based peptidomimetics, were found to be potent agents against hepatitis [5], AIDS [6] and SARS [7]. In addition, hydrazine derivatives have been used for derivatization of nanostructures [8]. Therefore, the synthesis of hydrazine derivatives is a matter of significant interest from both theoretical and practical perspectives. On the other hand, the promising usefulness of the benzamidomethyl derivatives as biologically active products [9,10,11] and their implication for pro-drug design [12,13,14,15] have been previously reported. Our good results with the benzamidomethylation of amines and hydrazides [16,17,18] using (benzamidomethyl)triethylammonium chloride (1), prompted us to synthesize some new products containing hydrazine and benzamidomethyl moieties with an aim to obtain new compounds with potential biological activity. In this short note, the synthesis of a novel tribenzamidomethyl hydrazine is reported.
Benzamidomethylation reaction of hydrazine, using 1 as a reagent, was performed smoothly in ethanol/aqueous media, under mild reaction conditions at ambient temperature (Scheme 1). The title compound (3) was obtained without using a catalyst. Crystals of the product were easily isolated by simple filtration and stored in a dark place at a temperature below 18 °C.
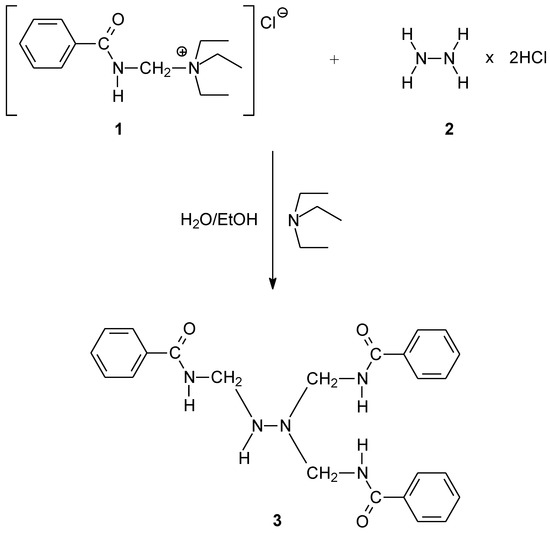
Scheme 1.
Synthetic route to the title compound 3.
The structure of the newly synthesized compound was confirmed by 1H NMR, 13C NMR, FTIR spectroscopy and high-resolution mass spectrometry as well.
Synthesis
Materials
All the reagents and solvents were obtained from commercial sources and used without further purification. (Benzamidomethyl)triethylammonium chloride was prepared according to the literature method [19]. Synthetic reaction was monitored by thin-layer chromatography with visualization by UV-light or staining with iodine vapors.
Instrumentation
Melting points were determined on a Reichert heating plate and are uncorrected. NMR spectra were recorded on a Bruker 250 MHz instrument using DMSO-d6 as solvent and tetramethylsilane as internal standard. Infrared spectra (KBr pellets) were measured on a Perkin-Elmer System 2000 FT-IR. The ESI mass spectra were recorded on a QTof premiere (Waters) equipped with an Acquity UPLC (Waters)." UPLC – Gradients and solvents: A = water + 0.1% formic acid; B = methanol + 0.1% formic acid; 0 min = 95% A; 2.5 min = 5% A, 6.5 min = 5% A; 6.6 min = 95% A, 8 min = 95% A.
Synthesis of tribenzamidomethyl hydrazine (3)
To a mixture of (benzamidomethyl)triethyl-ammonium chloride (465.1 mg, 1.72 mmol) in 8 mL of water and 0.2 mL of triethylamine, a solution of hydrazine dihydrochloride (59.8 mg, 0.57 mmol) in 8 mL of ethanol was added. Afterwards, the reaction mixture was stirred overnight at room temperature. In this phase, colorless crystals were obtained, which were collected by vacuum filtration. The purification was performed by dissolving the product in ethanol and precipitation with water.
Melting point of pure crystals: 123–125 °C. Typical yield 85%.
FTIR (KBr, cm−1): v(N-H) 3294, 3359, Amide I 1642, Amide II 1542;
1H-NMR (250 MHz, DMSO-d6): δ/ppm 8.81 (t, J = 6.0 Hz, 1H, CONHCH2NHN, overlap), 8.78 (t, J = 6.0 Hz, 2H, CONHCH2NN, overlap) 7.37–7.88 (m, 15H, ArH), 4.51 (t, J = 5.0 Hz, 1H, NNH), 4.36 (d, J = 6.0 Hz, 4H, CH2NN, overlap), 4.34 (t, J = 5.5 Hz, 2H, CH2NHN, overlap);
13C NMR (62.89 MHz, CDCl3): δ/ppm 167.34, 166.41 C=O; 55.68, 58.68 CH2; Ar: 134.41; 131.41, 131.29, 128.28;
HRMS (ESI, pos) m/z: 432.1900 (M+H)+; 454.1701 (M+Na)+.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We are grateful to the Macedonian Ministry of Education and Science (Contract 03-1586) and the Bulgarian National Science Fund (Contract BM-02/07) for financial support.
References and Notes
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Ling, L.; Urichuk, L.J.; Sloley, B.D.; Coutts, R.T.; Baker, G.B.; Shan, J.J.; Pang, P.K.T. Synthesis of N-propargylphenelzine and analogues as neuroprotective agents. Bioorg. Med. Chem. Lett. 2001, 11, 2715–2717. [Google Scholar] [CrossRef]
- Černuchová, P.; Vo-Thanh, G.; Milata, V.; Loupy, A.; Jantová, S.; Theiszová, M. Utilization of 2-ethoxymethylene-3-oxobutanenitrile in the synthesis of heterocycles possessing biological activity. Tetrahedron 2005, 61, 5379–5387. [Google Scholar]
- Rabong, C.; Jordis, U.; Phopase, J.B. NXO Building Blocks for Backbone Modification of Peptides and Preparation of Pseudopeptides. J. Org. Chem. 2010, 75, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Durkin, J.P.; Windsor, W.T. Azapeptides as inhibitors of the hepatitis C virus NS3 serine protease. Bioorg. Med. Chem. Lett. 2002, 12, 1005–1008. [Google Scholar] [CrossRef]
- Raja, A.; Lebbos, J.; Kirkpatrick, P. Fresh from the Pipeline: Atazanavir sulphate. Nat. Rev. Drug Discov. 2003, 2, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Cherney, M.M.; Huitema, C.; Liu, J.; James, K.E.; Powers, J.C.; Eltis, L.D.; James, M.N.G. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005, 353, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis, M.M.; Murata, Y.; Komatsu, K.; Orfanopoulos, M. Efficient synthesis of open-cage fullerene derivatives having 16-membered-ring orifices. J. Org. Chem. 2007, 72, 7042–7045. [Google Scholar] [PubMed]
- Tiwari, K.A.; Singh, K.V.; Bajpai, A.; Shukla, G.; Singh, S.; Mishra, K.A. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur. J. Med. Chem. 2007, 42, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Artico, M.; Valente, S.; Cerbara, I.; Befani, O.; Turini, P.; Vedova, D.L.; Agostinelli, E. Synthesis and biochemical evaluation of (R)-5-acyloxymethyl- and (S)-5-acylaminomethyl-3-(1H-pyrrol-1-yl)-2-oxazolidinones as new anti-monoamine oxidase (anti-MAO) agents. ARKIVOC 2004, 2004, 32–43. [Google Scholar]
- Zlotin, G.S.; Sharova, V.I.; Luk`yanov, A.O. Chemical properties of N-(amidomethy)- and N-(imidomethyl)glycine derivatives 2. Reactions at alkoxycarbonyl and carboxyl groups. Rus. Chem. Bul. 1996, 45, 1680–1687. [Google Scholar]
- Schioppacassi, G.; Morvillo, E.; Bruna, C.D.; Franceschi, G.; Foglio, M. In vitro and in vivo evaluation of benzamidomethyl-benzylpenicillinate (FI7303). A new 'repository' form. Chemotherapy 1978, 24, 338–342. [Google Scholar] [PubMed]
- Bundgaard, H.; Nielsen, N.M.; Buur, A. Aspirin prodrugs: synthesis and hydrolysis of 2-acetoxybenzoate esters of various N-(hydroxyalkyl) amides. Int. J. Pharm. 1988, 44, 151–158. [Google Scholar] [CrossRef]
- Moreira, R.; Calheiros, T.; Cabrita, J.; Mendes, E.; Pimentel, M.; Iley, J. Acyloxymethyl as a drug protecting group. Part 3. Tertiary O-Amidomethyl esters of penicillin G: Chemical hydrolysis and anti-bacterial activity. Pharm. Res. 1996, 13, 70–75. [Google Scholar] [PubMed]
- Getz, J.J.; Prankerd, R.J.; Sloan, K.B. Mechanism of hydrolysis of benzamidomethyl derivatives of phenols and its implications for prodrug design. J. Org. Chem. 1992, 57, 1702–1706. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride. 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acods. Molecules 2000, 5, 927–936. [Google Scholar]
- Popovski, E. Synthesis of N-(N’-benoylhydrazinomethyl)benzamide. Molbank 2007, 2007, M525. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-[N’-(2-hydroxy-2,2-diphenylacethyl)hydrazinomethyl]benzamide. Molbank 2007, 2007, M526. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple Method for Benzamidomethylation of Phenols in Water Solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).