(2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one
Abstract
:Introduction


Experimental
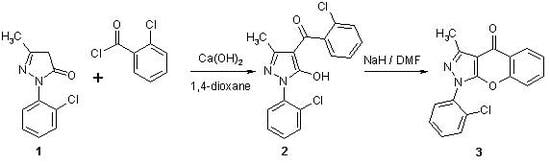
1-(2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications, 4th ed.; Thieme: Stuttgart, Germany, 2001; p. 99. [Google Scholar]
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An efficient approach to heterocyclic analogues of xanthone: A short synthesis of all possible Pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 4219–4229. [Google Scholar] [CrossRef]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and tetracyclic heteroaromatic systems: Synthesis of novel benzo-, benzothieno- and thieno-fused pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87–104. [Google Scholar] [CrossRef]
- Eller, G.A.; Holzer, W. A convenient approach to heterocyclic building blocks: Synthesis of novel ring systems containing a [5,6]pyrano[2,3-c]pyrazol-4(1H)-one moiety. Molecules 2007, 12, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4’,3’:5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: Synthesis and characterization of a novel tetracyclic ring system. J. Heterocycl. Chem. 2007, 44, 1139–1143. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of novel polycyclic ring systems containing two pyrano[2,3-c]pyrazol-4(1H)-one moieties. Chem. Heterocycl. Comp. 2007, 43, 1060–1064. [Google Scholar] [CrossRef]
- Eller, G.A.; Habicht, D.; Holzer, W. Synthesis of a novel pentacycle: 8-methyl-10-phenylpyrazolo[4’,3’:5,6]pyrano[3,2-c][1,10]phenanthrolin-7(10H)-one. Chem. Heterocycl. Comp. 2008, 44, 709–714. [Google Scholar] [CrossRef]
- Eller, G.A.; Zhang, Q.; Habicht, D.; Datterl, B.; Holzer, W. Synthesis and NMR data of pyrazolo[4’,3’:5,6]pyrano[2,3-b]pyrazin-4(1H)-ones: Derivatives of a novel tricyclic ring system. Acta Chim. Slov. 2009, 56, 521–526. [Google Scholar]
- Sarenko, A.S.; Kvitko, I.Y.; Efros, L.S. Heterocyclic analogs of xanthones. C-Acylation of 5-pyrazolones and synthesis of chromono[3,2-d]pyrazoles. Chem. Heterocycl. Comp. 1972, 8, 722–727. [Google Scholar] [CrossRef]
- Jensen, B.S. The synthesis of 1-phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and more basic NMR experiments: A practical course, 2nd edWiley–VCH: Weinheim, Germany, 1998. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Batezila, G.; Holzer, W. (2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank 2010, 2010, M661. https://doi.org/10.3390/M661
Batezila G, Holzer W. (2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank. 2010; 2010(1):M661. https://doi.org/10.3390/M661
Chicago/Turabian StyleBatezila, Giselle, and Wolfgang Holzer. 2010. "(2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one" Molbank 2010, no. 1: M661. https://doi.org/10.3390/M661
APA StyleBatezila, G., & Holzer, W. (2010). (2-Chlorophenyl)-3-methylchromeno[2,3-c]pyrazol-4(1H)-one. Molbank, 2010(1), M661. https://doi.org/10.3390/M661