Abstract
The palladium-catalyzed reaction of 4-bromo-3-methoxy-1-phenyl-1H-pyrazole with acrolein diethyl acetal gives the title compound in good yield. Detailed spectroscopic data (1H NMR, 13C NMR, 15N NMR, IR, MS) are presented.
Aromatic 2,3-unsaturated aldehydes are useful precursors in synthetic organic chemistry. For example, cinnamaldehyde was used as a starting material for the synthesis of various heterocyclic compounds, including tetrahydrothiophene [1], dihydropyridine [2], hexahydro-1H-pyrrolizine [3], benzo[a]quinolizidine [4], and pyrido[1,2-a]indole [5] derivatives.
In recent years efficient protocols for the preparation of aromatic 2,3-unsaturated aldehydes by Heck type palladium catalyzed cross-coupling reactions of arylhalides with acrolein diethylacetal [6,7,8] or 3,3-diacetoxypropene [9] have been developed. These methods allowed also to prepare (hetero)aromatic 2,3-unsaturated aldehydes containing pyridine, chinoline, isoquinoline, thiophene and benzothiophene nuclei [6,7,8,9].
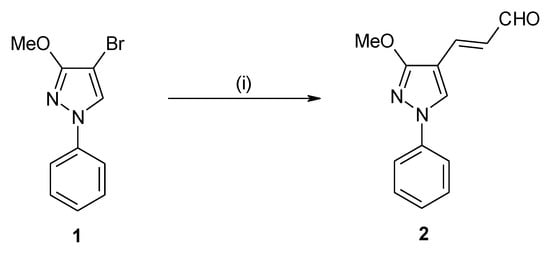
As a result of our interest in the synthesis of functionalized pyrazoles [10,11,12,13], we have developed a synthetic pathway to (2E)-3-(1-phenyl-3-methoxy-1H-pyrazol-4-yl)-2-propenal as a precursor to biologically active compounds and materials for non-linear optics.
As a starting material 4-bromo-3-methoxy-1-phenyl-1H-pyrazole (1) was used [14,15]. Palladium-catalyzed cross coupling of 1 with acrolein diethyl acetal afforded (2E)-3-(3-methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal (2). The structure of 2 was confirmed by its spectroscopic data (1H NMR, 13C, NMR, 15N NMR, IR, MS) as well as by elemental analysis. E-Configuration at the C=C double bond unequivocally follows from the magnitude of the vicinal coupling between the alkene protons H-2 and H-3 (3J(H2,H3) = 15.8 Hz).
Experimental
The melting point was determined on a Mel-Temp (Capillary Melting point Apparatus) and is uncorrected. Mass spectrum: Waters ZQ 2000 instrument (APCI+, 20 V). IR spectrum: Perkin Elmer Spectrum GX FT-IR System instrument (KBr-disc). The elemental analysis was performed with an Exeter Analytical CE-440 Elemental Analyzer. 1H and 13C NMR spectra were recorded on a Varian UnityPlus 300 spectrometer at 28 °C (299.95 MHz for 1H, 75.43 MHz for 13C). The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 7.26 ppm (1H in CDCl3) and δ = 77.0 ppm (13C in CDCl3). The digital resolutions were 0.2 Hz/data point in the 1H and 0.4 Hz/data point in the 1H-coupled 13C-NMR spectra (gated decoupling). A gradient selected 15N,1H-HMBC spectrum (50.68 MHz for 15N) was obtained on a Bruker Avance 500 instrument with a ‘directly’ detecting broadband observe probe (BBFO) and was referenced against external nitromethane.
(2E)-3-(3-Methoxy-1-phenyl-1H-pyrazol-4-yl)-2-propenal (2)
To a stirred solution of 4-bromo-3-methoxy-1-phenyl-1H-pyrazole (1) (506 mg, 2.0 mmol) in DMF (8 mL) acrolein diethyl acetal (0.9 mL, 6 mmol), nBu4NOAc (1.206 g, 4.0 mmol), K2CO3 (414 mg, 3.0 mmol), KCl (149 mg, 2 mmol), and Pd(OAc)2 (13 mg, 0.06 mmol) were added. The mixture was stirred for 4 days at 90 °C. After cooling, 2N HCl was slowly added and the reaction mixture was stirred at room temperature for 10 min. Then, it was diluted with ethyl acetate and washed with water. The organic layer was dried over anhydrous Na2SO4, filtered and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (silica gel, eluent: ethyl acetate–n-hexane, 1:4) to give 240 mg (53%) of 2 as colorless crystals, mp 131–133 ºC.
IR (KBr) ν (cm-1): 3109, 2727, 1675, 1618, 1567, 1508, 1421, 1239, 1173, 747, 633.
MS (EI, 70 eV): (m/z, %) 229 ([M+H]+, 100).
1H NMR (CDCl3, 300 MHz): δ (ppm) 4.07 (s, 3H, OMe), 6.71 (dd, 3J(H2,H3) = 15.8 Hz, 3J(H2,CHO) = 8.0 Hz, 1H, H-2), 7.27 (m, 1H, Ph H-4), 7.32 (d, 3J(H3,H2) = 15.8 Hz, 1H, H-3), 7.44 (m, 2H, Ph H-3,5), 7.62 (m, 2H, Ph H-2,6), 7.96 (s, 1H, pyrazole H-5), 9.56 (d, 3J(CHO,H2) = 8.0 Hz, 1H, CHO).
13C NMR (CDCl3, 75 MHz): δ (ppm) 56.5 (1J = 146.5 Hz, OMe), 106.3 (2J(pyrC4,pyrH5) = 7.4 Hz, 2J(pyrC4,H3) = 1.6 Hz, 3J(pyrC4,H2) = 5.3 Hz, pyrazole C-4), 118.1 (Ph C-2,6), 126.4 (Ph C-4), 127.3 (1J = 162.8 Hz, 2J(C2,CHO) = 25.0 Hz, 2J(C2,H3) = 2.6 Hz, H-2), 128.1 (1J = 186.9 Hz, 3J(pyrC5,H3) = 4.9 Hz, pyrazole C-5), 129.5 (Ph C-3,5), 139.2 (Ph C-1), 141.4 (1J = 152.5 Hz, 3J(C3,CHO) = 1.5 Hz, 3J(C3,pyrH5) = 1.5 Hz, C-3), 163.1 (3J(pyrC3,pyrH5) = 9.1 Hz, 3J(pyrC3,H3) = 6.8 Hz, 3J(pyrC3,OMe) = 5.3 Hz, pyrazole C-3), 193.8 (1J = 169.5 Hz, 2J(CO,H2) = 1.7 Hz, 3J(CO,H3) = 9.3 Hz, CHO).
15N NMR (CDCl3, 50.7 MHz): δ (ppm) –182.5 (pyrazole N-1), –118.5 (pyrazole C-2).
Anal. Calcd for C13H12N2O2: C, 68.41%; H, 5.30%; N, 12.27%. Found: C, 68.30%; H, 5.49%; N, 12.24%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Juárez, E.; García, A.; Hommer, H.; Salas, M.; Gordillo, B. Stereoselective synthesis and conformational analysis of aromatic C-thionucleosides. Heteroatom Chem. 2006, 17, 289–298. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, X.X.; Rao, Q.Q.; Gong, L.Z. Organocatalytic asymmetric three-component cyclization of cinnamaldehydes and primary amines with 1,3-dicarbonyl compounds: Straightforward access to enantiomerically enriched dihydropyridines. Angew. Chem. Int. Ed. 2008, 47, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.C.; Liu, K.L.; Tsai, C.W.; Liao, J.H. Proline-mediated dimerization of cinnamdehydes via 1,3-dipolar cycloaddition reaction with azomethine ylides. A rapid access to highly functionalized hexahydro-1H-pyrrolizine. Tetrahedron Lett. 2008, 49, 5480–5483. [Google Scholar] [CrossRef]
- Jiang, J.; Qing, J.; Gong, L.Z. Asymmetric synthesis of 3-amino-δ-lactams and benzo[a]quinolizines by catalytic cyclization reactions involving azlactones. Chem. Eur. J. 2009, 15, 7031–7034. [Google Scholar] [CrossRef] [PubMed]
- Shachkus, A.A.; Degutis, Y.A. Synthesis of 8-phenyl-10H-pyrido[1,2-a]indole salts from 2,3,3-trimethyl-3H-indole chlorides with cinnamaldehyde. Chem. Heterocycl. Comp. 1987, 23, 401–403. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Cacchi, S.; Fabrizi, G. An efficient palladium-catalyzed synthesis of cinnamaldehydes from acrolein diethyl acetal and aryl iodides and bromides. Org. Lett. 2003, 5, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Noël, S.; Luo, C.; Pinel, C.; Djakovitch, L. Efficient heterogeneously palladium-catalysed Heck arylation of acrolein diethyl acetal. Synthesis of cinnamaldehydes or 3-arylpropionic esters. Adv. Synth. Catal. 2007, 349, 1128–1140. [Google Scholar] [CrossRef]
- Alacid, E.; Najera, C. Acrolein diethyl acetal: A three-carbon homolating reagent for the synthesis of β-arylpropanoates and cinnamaldehydes by Heck reaction catalyzed by a Kaiser oxime resin derived palladacycle. Eur. J. Org. Chem. 2008, 18, 3102–3106. [Google Scholar] [CrossRef]
- Berthiol, F.; Doucet, H.; Santelli, M. Direct synthesis of cinnamaldehyde derivatives by reaction of aryl bromides with 3,3-diacetoxypropene catalyzed by a palladium-tetraphosphine complex. Catalysis Letters 2005, 102, 281–284. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G.A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar] [CrossRef]
- Holzer, W.; Krca, I. New 1-substituted 4-cinnamoyl-5-hydroxypyrazoles and precursors thereof: Synthesis, ring closure reactions and NMR-spectroscopic investigations. Heterocycles 2003, 60, 2323–2342. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Huber, T. Ein effizienter Zugang zu aryl- oder benzyl-4-pyrazolylketonen und carbinolen. Arch. Pharm. (Weinheim) 1987, 320, 1267–1272. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Obala, C. Beiträge zur Chemie von Pyrazolylalkinen. Monatsh. Chem. 1988, 119, 253–262. [Google Scholar] [CrossRef]
- Koenig, H.; Goetz, N.; Klein, U.; Eller, K. Process for producing N-substituted 3-hydroxypyrazoles. Chem. Abstr. 1997, 126. 199566, WO 9703969. [Google Scholar]
- Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. 4-Bromo-3-methoxy-1-phenyl-1H-pyrazole. Molbank 2009, M639. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).