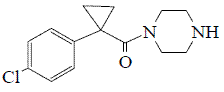
[1-(4-Chlorophenyl)cyclopropyl](piperazin-1-yl)methanone
Abstract
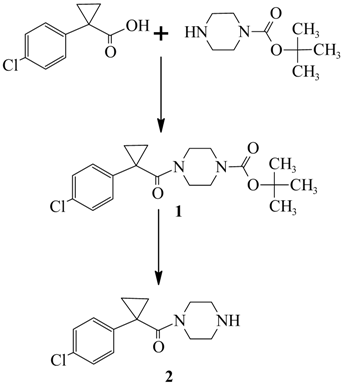
:
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Singh, R.; Geetanjali; Chauhan, S.M.S. 9,10-Anthraquinones and other biologically active compounds from the genus rubia. Chem Biodivers. 2004, 1, 1241–1264. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, P.M.; Boutwell, R.K. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters, Part 2. Crit. Rev. Toxicol. 1981, 8, 199–234. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Tyler, M.I.; Howden, M.E.H. Synthesis and structure-activity studies of potential antitumor agents derived from esters of phorbol. Aust. J. Chem. 2002, 40, 193–200. [Google Scholar] [CrossRef]
- Patel, H.S.; Desai, H.D.; Mistry, H.J. Synthesis and antimicrobial activity of some new piperazine derivaties containing aryl sulfonyloxy group. E-J. Chem. 2004, 1, 93–98. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hobbs, C.F.; Wengaten, H. Titanium tetrachloride promoted condensations of amines with carboxamides and similar species. J. Org. Chem. 1970, 35, 1542–1547. [Google Scholar] [CrossRef]
- Burnell-Gurty, C.; Roskamp, E.J. The conversion of carboxylic acids to amides via tin(II) reagents. Tetrahedron Lett. 1993, 34, 5193–5196. [Google Scholar] [CrossRef]
- Froyen, P. The conversion of carboxylic acids into amides via NCS/triphenylphosphine. Synth. Commun. 1995, 25, 959–962. [Google Scholar] [CrossRef]
- Balalaie, S.; Mahdidoust, M.; Eshaghi-Najafabadi, R. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate as an efficient coupling reagent for the amidation and phenylhydrazation of carboxylic acids at room temperature. J. Iran. Chem. Soc. 2007, 4, 364–369. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Padmashali, B.; Mallikarjuna, S.M.; Chidananda, B.N. [1-(4-Chlorophenyl)cyclopropyl](piperazin-1-yl)methanone. Molbank 2009, 2009, M641. https://doi.org/10.3390/M641
Padmashali B, Mallikarjuna SM, Chidananda BN. [1-(4-Chlorophenyl)cyclopropyl](piperazin-1-yl)methanone. Molbank. 2009; 2009(4):M641. https://doi.org/10.3390/M641
Chicago/Turabian StylePadmashali, Basavaraj, Siddapura M. Mallikarjuna, and Ballekere N. Chidananda. 2009. "[1-(4-Chlorophenyl)cyclopropyl](piperazin-1-yl)methanone" Molbank 2009, no. 4: M641. https://doi.org/10.3390/M641
APA StylePadmashali, B., Mallikarjuna, S. M., & Chidananda, B. N. (2009). [1-(4-Chlorophenyl)cyclopropyl](piperazin-1-yl)methanone. Molbank, 2009(4), M641. https://doi.org/10.3390/M641