6-(4-(2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}-ethyl)-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione)
Abstract
:1. Introduction

2. Experimental
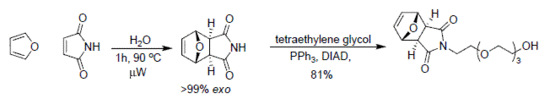
2.1. endo/exo-7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboximide: Conventional method
2.2. exo-7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboximide: Microwave method
2.3. Mitsunobu alkylation of the exo-imide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Breitenkamp, K.; Emrick, T. Amphiphilic Ruthenium Benzylidene Metathesis Catalyst with PEG-Substituted Pyridine Ligands. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 5715–5721. [Google Scholar] [CrossRef]
- Kwart, H.; Burchuk, I. Isomerism and Adduct Stability in the Diels-Alder Reaction: The Adducts of Furan and Maleimide. J. Am. Chem. Soc. 1952, 74, 3094–3097. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Rauscher, H.; Behm, R.J.; Goedel, W.A. Maleimido-Terminated Self-Assembled Monolayers. Chem. Eur. J. 2005, 11, 3968–3978. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.K.; Weber, R.; Tweig, R.J. Improved Synthesis of DCDHF Fluorophores with Maleimide Functional Groups. Tetrahedron Lett. 2006, 47, 7213–7217. [Google Scholar] [CrossRef] [PubMed]
- Rulisek, L.; Sebek, P.; Havlas, V.; Hrabal, R.; Capek, P.; Svatos, A. An Experimental and Theoretical Study of Selectivity of Furan-Maleic Anhydride and Furan-Maleimide Diels-Alder Reactions. J. Org. Chem. 2005, 70, 6295–6302. [Google Scholar] [CrossRef] [PubMed]
- Kristof, K.; Marijan, K. Microwave-Assisted Diels-Alder Reaction of 2H-Pyran-2-ones with Maleimides Towards Fused Bicyclo[2.2.2]octenes. Heterocycles 2007, 73, 481–491. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gooden, D.M. 6-(4-(2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}-ethyl)-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione). Molbank 2009, 2009, M638. https://doi.org/10.3390/M638
Gooden DM. 6-(4-(2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}-ethyl)-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione). Molbank. 2009; 2009(4):M638. https://doi.org/10.3390/M638
Chicago/Turabian StyleGooden, David M. 2009. "6-(4-(2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}-ethyl)-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione)" Molbank 2009, no. 4: M638. https://doi.org/10.3390/M638
APA StyleGooden, D. M. (2009). 6-(4-(2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}-ethyl)-10-oxa-4-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione). Molbank, 2009(4), M638. https://doi.org/10.3390/M638