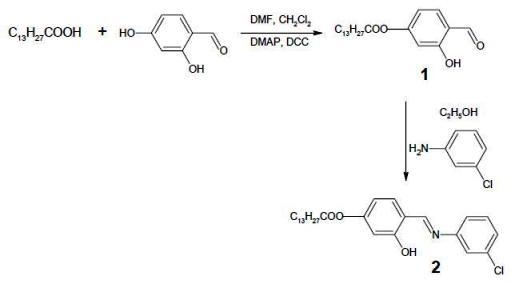
4-{[(3-Chlorophenyl)imino]methyl}-3-hydroxyphenyl Myristate
Abstract
:Introduction
Synthesis
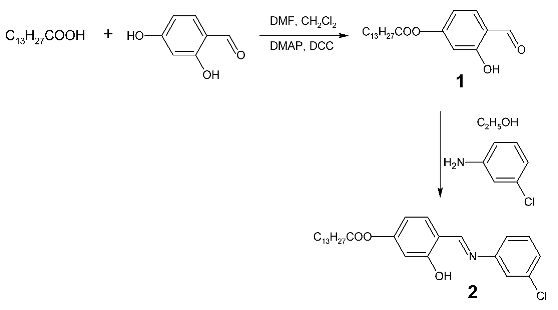
Preparation of 4-formyl-3-hydroxyphenyl myristate, 1
Preparation of 4-{[(3-chlorophenyl)imino]methyl}-3-hydroxyphenyl myristate, 2
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Hadjoudis, E.; Vittorakis, M.; Moustakali-Mavridis, I. Photochromism and thermochromism of schiff bases in the solid state and in rigid glasses. Tetrahedron 1987, 43, 1345–1360. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Rontoyianni, A.; Ambroziak, K.; Dziembowska, T.; Mavridis, I.M. Photochromism and thermochromism of solid trans-N,N'-bis(salicylidene)-1,2-cyclohexanediamines and trans-N,N'-bis-(2-hydroxynaphylidene)-1,2-cyclohexanediamine. J. Photochem. Photobiol. A: Chem. 2004, 162, 521–530. [Google Scholar] [CrossRef]
- Oshima, A.; Momotake, A.; Arai, T. Photochromism, thermochromism, and solvatochromism of naphthalene-based analogues of salicylideneaniline in solution. J. Photochem. Photobiol. A: Chem. 2004, 162, 473–479. [Google Scholar] [CrossRef]
- Suzuki, T.; Arai, T. Novel insight into the photochromism and thermochromism of hydrogen bonded schiff bases. Chem. Lett. 2001, 124–125. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Ishizawa, N.; Suda, K.; Boey, P.L.; Mahmood, W.A.K. Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J. Mol. Struct. 2003, 658, 87–99. [Google Scholar] [CrossRef]
- Sudhakar, S.; Narasimhaswamy, T.; Srinivasan, K.S.V. Synthesis, characterization and thermal properties of 4,4'-bis(4-n-alkoxybenzoyloxy)benzylideneanilines and bis(4-benzylidene-4-n-alkoxyaniline) terephthalates. Liq. Cryst. 2000, 27, 1525–1532. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, S.-T.; Ong, S.-T.; Chong, Y.-T.; Yeap, G.-Y. 4-{[(3-Chlorophenyl)imino]methyl}-3-hydroxyphenyl Myristate. Molbank 2009, 2009, M629. https://doi.org/10.3390/M629
Ha S-T, Ong S-T, Chong Y-T, Yeap G-Y. 4-{[(3-Chlorophenyl)imino]methyl}-3-hydroxyphenyl Myristate. Molbank. 2009; 2009(4):M629. https://doi.org/10.3390/M629
Chicago/Turabian StyleHa, Sie-Tiong, Siew-Teng Ong, Yee-Ting Chong, and Guan-Yeow Yeap. 2009. "4-{[(3-Chlorophenyl)imino]methyl}-3-hydroxyphenyl Myristate" Molbank 2009, no. 4: M629. https://doi.org/10.3390/M629
APA StyleHa, S.-T., Ong, S.-T., Chong, Y.-T., & Yeap, G.-Y. (2009). 4-{[(3-Chlorophenyl)imino]methyl}-3-hydroxyphenyl Myristate. Molbank, 2009(4), M629. https://doi.org/10.3390/M629