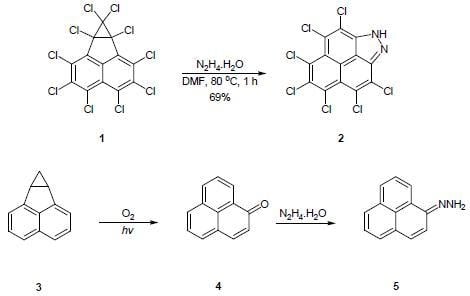
3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole
Abstract
:
Experimental Section
3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Weeratunga, G.; Austrup, M.; Rodrigo, R. Preparation, properties, and some chemical reactions of phenaleno[1,9-bc]furan. J. Chem. Soc., Perkin Trans. 1 1988, 3169–3174. [Google Scholar]
- Tominaga, Y.; Lee, M.L.; Castle, R.N. Synthesis of phenaleno[1,9-bc]thiophene. J. Heterocycl. Chem. 1981, 18, 977–979. [Google Scholar] [CrossRef]
- Otsubo, T. Novel heteroarenes forming conducting molecular complexes. Synlett 1997, 544–550. [Google Scholar] [CrossRef]
- Imamura, K.; Hirayama, D.; Yoshimura, H.; Takimiya, K.; Aso, Y.; Otsubo, T. Application of flash vacuum pyrolysis to the synthesis of sulfur-containing heteroaromatic systems. Tetrahedron Lett. 1999, 40, 2789–2792. [Google Scholar] [CrossRef]
- Haddon, R.C.; Chichester, S.V.; Stein, S.M.; Marshall, J.H.; Mujsce, A.M. Perchloro-7H-cycloprop[a]acenaphthylene and the perchlorophenalenyl system. J. Org. Chem. 1987, 52, 711–712. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Chen, Y.; Cao, Y.; Best, T.P.; Itkis, M.E.; Beer, L.; Oakley, R.T.; Cordes, A.W.; Brock, C.P.; Haddon, R.C. Perchlorophenalenyl radical. J. Am. Chem. Soc. 2001, 123, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Pagni, R.M.; Burnett, M.N.; Hassaneen, H.M. The behavior of the singlet and triplet spin states of methylene-bridged 1,8-naphthoquinodimethane with O2. Tetrahedron 1982, 38, 843–851. [Google Scholar] [CrossRef]
- Lock, G.; Gergely, G. Über Perinaphthinden. Chem. Ber. 1944, 77, 461–465. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Demetriadou, P.K.; Koutentis, P.A. 3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole. Molbank 2009, 2009, M625. https://doi.org/10.3390/M625
Demetriadou PK, Koutentis PA. 3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole. Molbank. 2009; 2009(4):M625. https://doi.org/10.3390/M625
Chicago/Turabian StyleDemetriadou, Polina K., and Panayiotis A. Koutentis. 2009. "3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole" Molbank 2009, no. 4: M625. https://doi.org/10.3390/M625
APA StyleDemetriadou, P. K., & Koutentis, P. A. (2009). 3,4,5,6,7,8,9-Heptachlorophenaleno[1,9-bc]pyrazole. Molbank, 2009(4), M625. https://doi.org/10.3390/M625