Abstract
Two new Cα-tetrasubstituted α-amino acids (2R,3S)-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2-(4-bromophenyl)-3-(tert-butoxycarbonylamino)-tetrahydrofuran-3-carboxylate and (2S,3R)-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2-(4-bromophenyl)-3-(tert-butoxycarbonylamino)-tetrahydrofuran-3-carboxylate were synthesized and characterized by NMR, MS, elemental analysis and X-ray.
Introduction
Cα-Tetrasubstituted α-amino acids are important building blocks for the construction of active peptidomimetics [1,2,3,4,5]. They are commonly used to introduce constrains into a peptide backbone through the stable quaternary α-carbon and attached substituents. Even larger geometrical constraints arise from covalent linkage of the substituents at the α-carbon with each other leading to cyclic Cα-tetrasubstituted α-amino acids. Such constrains may alter the chemical reactivity of the surrounding functional groups and reduce e.g. the hydrolysis rate of amide bonds or ester groups [6,7]. We have recently developed a new synthetic route to cyclic Cα-tetrasubstituted tetrahydrofuran amino acids (TAAs) [8]. The unnatural amino acids are readily accessible in a four step synthesis from commercially available starting materials, bear a stable quaternary stereocenter at the α-carbon and a second stereocenter at their β-carbon. However, the unnatural TAAs are obtained in racemic form due to the deprotonation of the α-carbon destroying stereochemical information in this position. To overcome this limitation, methionine (-)-menthol esters were used as starting material yielding diastereomers separable by silica gel column chromatography.
Synthesis
Boc-protected methionine 1 was converted with (-)-menthol into its ester 6 using a Steglich-type esterification reaction. Methylation resulted in the sulfonium salt 2. The cyclisation reaction was performed using 4-bromobenzaldehyde 3. The reaction gave selectively the trans-products (4, 5) in an overall yield of 69 %. The two resulting trans-diastereomers 4 and 5 were separated by flash column chromatography.

The configuration of compound 5 was confirmed by an X-ray structure analysis. Suitable crystals were obtained by recrystallization from MeOH/EtOH 8:1.
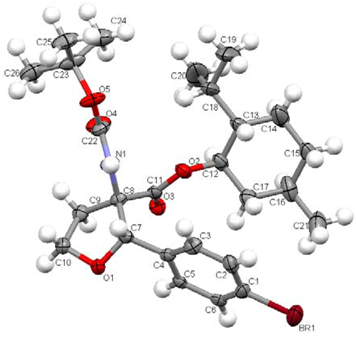

(S)-(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(tert-butoxycarbonylamino)-4-(methylthio) butanoate (6)
Boc-Met-OH 1 (10.0 g, 40.1 mmol) was dissolved under a nitrogen atmosphere in 200 ml of dry DCM and cooled to 0 °C in an ice bath. To this solution DMAP (408 mg, 3.34 mmol) and (-)menthol (7.52 ml, 48.1 mmol) were added. Under vigorous stirring N,N’-dicyclohexylcarbodiimide (10.8 g, 52.1 mmol) was slowly added in portions. The mixture was stirred at 0 °C for 2 hours, allowed to warm to room temperature and stirred for additional 12 hours. The urea was filtered off and washed twice with 50 ml of DCM. The solvent was evaporated under reduced pressure. The crude product was then purified by column chromatography on silica gel (PE:diethyl ether 80:20, Rf = 0.20) to give the product as colorless solid (13.3 g, 34.3 mmol, 86 %).

MP 59-60 °C.
MS (CI, NH3): m/z (%) = 405.2 (100) [MNH4+], 388.2 (54) [MH+], 349.1 (45) [MNH4+ - C4H8], 288.2 (16) [MH+ - Boc].
IR (NEAT) [cm-1]: = 3345, 2927, 2970, 2121, 1703, 1508, 1458, 1365, 1310, 1275, 1247, 1227, 1152, 1055, 1001, 960, 866, 789.
1H-NMR (300 MHz, CDCl3): δ = 0.74 (d, 3JH,H = 6.9, 3 H, 19), 0.88 (d, 3JH,H = 4.4, 3 H, 21), 0.90 (d, 3JH,H = 4.1, 3 H, 21), 0.92-1.26 (m, 3 H), 1.32-1.65 (m, 11 H), 1.63-1.75 (m, 2 H), 1.79 (2.04 (m, 3 H), 2.07-2.18 (m, 4 H), 2.45-2.61 (m, 2 H, 16), 4.36 (dd, 3JH,H = 7.0, 3JH,H = 12.5, 1 H, 6), 4.73 (dt, 3JH,H = 4.4, 3JH,H = 11.0, 1 H, 9), 4.36 (d, 3JH,H = 5.1, 1 H, 5).
13C-NMR (75 MHz, CDCl3): δ = 15.5 (+, 1 C, 21), 16.2 (+, 1 C, 21), 20.8 (+, 1 C, 18), 22.0 (+, 1 C, 19), 23.3 (-, 1 C, 13), 26.1 (+, 1 C, 20), 28.3 (+, 3 C, 1), 29.9 ( -, 1 C, 16), 31.4 (+, 1 C, 11), 32.6 (-, 1 C, 15), 34.1 (-, 1 C, 12), 40.7 (-, 1 C, 10), 46.9 (+, 1 C, 14), 53.0 (+, 1 C, 6), 75.6 (+, 1 C, 9), 79.9 (Cquat, 1 C, 2), 155.3 (Cquat, 1 C,4), 171.8 (Cquat, 1 C, 7). - 30.1 (-, 1 C, 9), 53.8 (+, 1 C, 6), 70.0 (-, 1 C, 17), 80.1 (Cquat, 1 C, 2), 114.9 (+, 1 C, 14), 128.8 (+, 2 C, 19/20) , 128.9 (+, 2 C, 19/20), 129.2 (Cquat, 1 C, 21), 133.9 (Cquat, 1 C, 10), 136.8 (+, 1 C, 12), 139.6 (Cquat, 1 C, 18), 148.4 (Cquat, 1 C, 15), 173.9 (Cquat, 1 C, 7).
Elemental analysis calcd. (%) for C20H37NO4S (387.58): C 61.98, H 9.62, N 3.61; found: C 61.81, H 9.33, N 3.47.
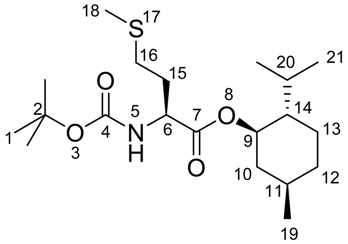
(S)-(3-(tert-Butoxycarbonylamino)-4-((1S,2S,5R)-2-isopropyl-5-methylcyclohexyloxy)-4-oxobutyl) di-methylsulfonium iodide (2)
(S)-(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(tert-butoxycarbonylamino)-4-(methylthio) butanoate 6 (5.00 g, 12.9 mmol) was dissolved in 39 ml of methyl iodide (3 ml/mmol) and stirred for three days at room temperature in the dark. The solution was cooled to 0 °C in an ice bath and 39 ml of heptane (3 ml/mmol) were added and the methyl iodide was evaporated under reduced pressure until the product started to precipitate. The mixture was kept at 0 °C in the dark for four hours to complete precipitation. The hygroscopic and light sensitive product was obtained after filtration and washing with ice-cold heptane as a colorless solid (10.8 g, 5.71 mmol, 84%) in analytically pure form.

MP 42 °C.
MS (ES, DCM/MeOH + 10 mmol/l NH4OAc): m/z (%) = 402.3 (100) [M+], 931.6 (4) [2M+ + I-].
IR (NEAT) [cm-1]: = 2957, 2925, 2870, 2361, 1704, 1508, 1456, 1366, 1310, 1274, 1246, 1161, 1048, 982, 784.
1H-NMR (300 MHz, CDCl3): δ = 0.68 (d, 3JH,H = 6.9, 3 H, 19), 0.81-0.88 (m, 7 H, 2 CH3 + CH), 0.92-1.16 (m, 2 H, CH2), 1.14-1.23 (m, 2 H, CH2), 1.38 (s, 9 H, CH3), 1.56-1.69 (m, 2H, CH2), 1.71-1.84 (m, 1 H, CH), 1.85-1,95 (m, 1 H, CH), 2.13-2.41 (m, 2 H, CH2), 3.61-3.79 (m, 1 H, CH2), 3.88-4.03 (m, 1 H, CH2), 4.11-4.36 (m, 1 H, CH), 4.69 (dt, 3JH,H = 4.5, 3JH,H = 10.8, 1 H, CH), 5.62 (d, 3JH,H = 5.8, 1 H, NH).
13C-NMR (75 MHz, CDCl3): δ = 14.1 (+, 1 C, CH3), 16.2 (+, 1 C, CH), 20.8 (+, 1 C, CH3), 22.0 (+, 1 C, CH3), 22.7 (-, 1 C, CH2), 23.1 (-, 1 C, CH2), 25.9 (+, 1 C, CH3), 26.1 (+, 1 C, CH3), 28.3 (+, 3 C, CH3), 31.4 (+, 1 C, CH), 31.9 (- 1 C, CH2), 34.0 (-, 1 C, CH2), 40.7 (-, 1 C, CH2), 46.7 (+, 1 C, CH), 52.1 (+, 1 C, CH), 76.6 (-, 1 C, CH), 80.8 (Cquat, 1 C, C(CH3)3), 155.9 (Cquat, 1 C, CO), 170.1 (Cquat, 1 C, CO).
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(4-bromophenyl)-3-(tert-butoxycarbonyl-amino)-tetra-hydrofuran-3-carboxylate (4/5)
In an oven dried Schlenk flask under nitrogen atmosphere the sulfonium salt 2 (601 mg, 1.14 mmol, 1.4 eq.) was dissolved in dry acetonitrile (5 ml per 1 mmol sulfonium salt). The colorless solution was cooled to -6 °C and potassium hydroxide (64 mg, 1.14 mmol, 1.4 eq.) followed by 4-bromobenzaldehyde 3 (150 mg, 0.81 mmol, 1 eq.) were added. The mixture was stirred at -6 °C for 5 hours. After complete consumption of starting material the reaction mixture was quenched with water (4 ml per mmol sulfonium salt) and was extracted with diethyl ether (1 x 4 ml/mmol, 2 x 5 ml/mmol sulfonium salt). The combined organic layers were washed with brine solution and dried over MgSO4. After removal of the solvent under reduced pressure the crude product was purified by flash chromatography using a 90:10 mixture of PE:diethyl ether (Rf = 0.10, 0.17) to give the separated compounds 4 (152 mg, 0.29 mmol) and 5 (141 mg, 0.27 mmol) in an overall yield of 69 %.
(2R,3S)-((1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl) 2-(4-bromophenyl)-3-(tert-butoxycarbonylamino)-tetrahydrofuran-3-carboxylate (4)
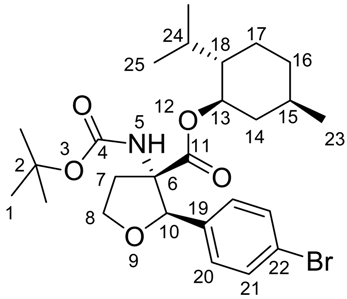
MP 52-53 °C.
MS (ES, DCM/MeOH + 10 mmol/l NH4OAc): m/z (%) = 524.3 (29) [MH+], 543.4 (100) [M + NH4+], 524.4 (100) [M – H+], 582.4 (81) [M + CH3COO-].
IR (NEAT) [cm-1]: = 2957, 2930, 2870, 1714, 1591, 1485, 1367, 1269, 1160, 1103, 1069, 1011, 847, 757
1H-NMR (600 MHz, COSY, CDCl3): δ = 0.44 (q, 3JH,H = 11.5, 1 H, 14), 0.61 (d, 3JH,H = 6.6, 3 H, 23), 0.69-0.78 (m, 1 H, 16), 0.80 (d, 3JH,H = 6.6, 3 H, 25), 0.82 (d, 3JH,H = 7.0, 3 H, 25), 0.86-0.94 (m, 1 H, 17), 1.15-1.22 (m, 1 H, 14), 1.24-1.30 (m, 2 H, 15 + 18), 1.48 (s, 9 H, 1), 1.52-1.62 (m, 3 H,16 + 17 + 24), 2.60-2.67 (m, 1 H, 7), 2.68-2.76 (m, 1 H, 7), 4.25-4.33 (m, 2 H, 8), 4.41 (dt, 3JH,H = 4.4, 3JH,H = 10.9, 1 H, 13), 5.12 (s, 1 H, 10), 5.71 (s, 1 H, 5), 7.20 (d, 3JH,H = 8.3, 2 H, 20), 7.40 (d, 3JH,H = 8.6, 2 H, 21).
13C-NMR (150 MHz, HSQC, HMBC, CDCl3): 15.7 (+, 1 C, 23), 20.9 (+, 1 C, 25), 21.9 (+, 1 C, 25), 22.8 (-, 1 C, 17), 25.8 (+, 1 C, 24), 28.4 (+, 3 C, 1), 31.1 (+, 1 C, 15), 33.9 (-, 1 C, 16), 35.6 (-, 1 C, 7), 39.8 (-, 1 C, 14), 46.7 (+, 1 C, 18), 67.8 (-, 1 C, 8), 69.4 (Cquat, 1 C, 6), 76.5 (+, 1 C, 13), 80.1 (Cquat, 1 C, 3), 84.0 (+, 1 C, 10), 121.8 (Cquat, 1 C, 22), 127.7 (+, 2 C, 20), 131.2 (+, 2 C, 21), 136.5 (Cquat, 1 C, 19), 154.3 (Cquat, 1 C, 4), 171.0 (Cquat, 1 C, 11).
Elemental analysis calcd. (%) for C26H38BrNO5 (524.49): C 59.42, H 7.08, N 2.66; found: C 59.54, H 7.30, N 2.67.
(2S,3R)-((1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl) 2-(4-bromophenyl)-3-(tert-butoxycarbonylamino)-tetrahydrofuran-3-carboxylate (5)
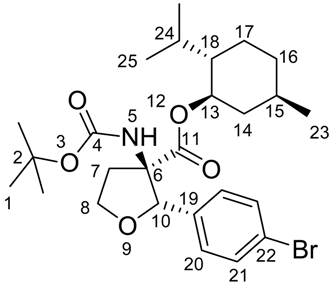
MP 88-90 °C.
MS (ES, DCM/MeOH + 10 mmol/l NH4OAc): m/z (%) = 524.3 (29) [MH+], 543.4 (100) [M + NH4+], 524.4 (100) [M – H+], 582.4 (81) [M + CH3COO-].
IR (NEAT) [cm-1]: = 2956, 2927, 2869, 1708, 1591, 1487, 1367, 1247, 1160, 1011, 1070, 1011, 847, 757.
1H-NMR (600 MHz, COSY, CDCl3): δ = 0.35-0.46 (m, 1 H, 14), 0.59 (d, 3JH,H = 6.6, 3 H, 23), 0.72-0.76 (m, 1 H, 16), 0.79 (d, 3JH,H = 6.9, 3 H, 25), 0.81 (d, 3JH,H = 6.4, 3 H, 25), 0.88-0.92 (m, 1 H, 17), 1.16-1.23 (m, 2 H, 14 + 18), 1.25-1.32 (m, 1 H, 15), 1.47 (s, 9 H, 1), 1.50-1.55 (m, 1 H, 24), 1.56-1.58 (m, 1 H, 16), 1.58-1.61 (m, 1 H, 17), 2.62-2.71 (m, 1 H, 7), 2.80 (dt, 3JH,H = 8.3, 3JH,H = 13.0, 1 H, 7), 4.18 (q, 3JH,H = 8.1, 1 H, 8), 4.34 (dt, 3JH,H = 4.2, 3JH,H = 8.4, 1 H, 8), 4.40 (dt, 3JH,H = 4.4, 3JH,H = 10.8, 1 H, 13), 4.97 (s, 1 H, 10), 5.46 (s, 1 H, 5), 7.19 (d, 3JH,H = 8.4, 2 H, 20), 7.41 (d, 3JH,H = 8.4, 2 H, 21).
13C-NMR (150 MHz, HSQC, HMBC, CDCl3): 15.6 (+, 1 C, 23), 21.0 (+, 1 C, 25), 22.0 (+, 1 C, 25), 22.7 (-, 1 C, 17), 25.2 (+, 1 C, 24), 28.3 (+, 3 C, 1), 31.1 (+, 1 C, 15), 33.9 (-, 1 C, 16), 35.8 (-, 1 C, 7), 39.7 (-, 1 C, 14), 46.6 (+, 1 C, 18), 67.7 (-, 1 C, 8), 69.7 (Cquat, 1 C, 6), 76.3 (+, 1 C, 13), 80.5 (Cquat, 1 C, 3), 85.2 (+, 1 C, 10), 122.1 (Cquat, 1 C, 22), 128.1 (+, 2 C, 20), 131.3 (+, 2 C, 21), 136.2 (Cquat, 1 C, 19), 154.3 (Cquat, 1 C, 4), 170.3 (Cquat, 1 C, 11).
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Hirschmann, R. Angew. Chem. Int. Ed. 1991, 30, 1278–1301.
- Hruby, V.J.; Al-Obeidi, F. Biochem. J. 1990, 268, 249–262. [PubMed]
- Rizo, J.; Gierasch, L.M. Annu. Rev. Biochem. 1992, 61, 387–418. [PubMed]
- DeGrado, W.F. Adv. Protein Chem. 1988, 39, 51–124. [PubMed]
- Rose, G.D.; Gierasch, L.M.; Smith, J.A. Adv. Protein Chem. 1985, 37, 1–109. [PubMed]
- Maity, P.; Konig, B. Biopolymers 2008, 90, 8–27. [PubMed]
- Aschi, M.; Lucente, G.; Mazza, F.; Mollica, A.; Morera, E.; Nalli, M.; Paradisi, M.P. Org. Biomol. Chem. 2003, 1, 1980–1988. [PubMed]
- Maity, P.; Zabel, M.; König, B.J. J. Org. Chem. 2007, 72, 8046–8053. [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
