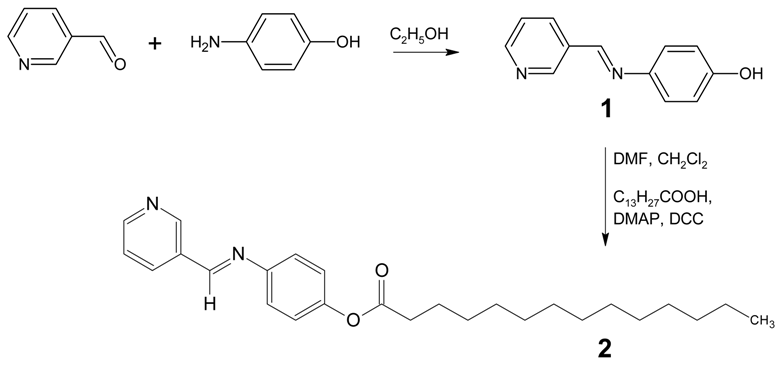
4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate
Abstract
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Hadjoudis, E.; Vittorakis, M.; Moustakali-Mavridis, I. Tetrahedron 1987, 43, 1345–1360.
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Sanehisa, S. Mol. Cryst. Liq. Cryst. 2004, 423, 73–84.
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Vill, V. Mol. Cryst. Liq. Cryst. 2006, 452, 63–72.
- Yeap, G.Y.; Ha, S.T.; Boey, P.L.; Mahmood, W.A.K.; Ito, M.M.; Youhei, Y. Mol. Cryst. Liq. Cryst. 2006, 452, 73–90.
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Youhei, Y. Liq. Cryst. 2006, 33, 205–211.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2008, 3, M582.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, S.-T.; Ong, L.-K.; Win, Y.-F.; Koh, T.-M.; Yeap, G.-Y. 4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate. Molbank 2009, 2009, M585. https://doi.org/10.3390/M585
Ha S-T, Ong L-K, Win Y-F, Koh T-M, Yeap G-Y. 4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate. Molbank. 2009; 2009(1):M585. https://doi.org/10.3390/M585
Chicago/Turabian StyleHa, Sie-Tiong, Lay-Khoon Ong, Yip-Foo Win, Teck-Ming Koh, and Guan-Yeow Yeap. 2009. "4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate" Molbank 2009, no. 1: M585. https://doi.org/10.3390/M585
APA StyleHa, S.-T., Ong, L.-K., Win, Y.-F., Koh, T.-M., & Yeap, G.-Y. (2009). 4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate. Molbank, 2009(1), M585. https://doi.org/10.3390/M585



