1. Introduction
In connection with our investigation of the competitive mesolytic cleavages [1] of radical anions we have prepared several symmetrically substituted 2,7-bis(halomethyl)naphthalene derivatives. The starting material for these derivatives was 2,7-bis(bromomethyl)naphthalene 1, which could be conveniently obtained by benzylic bromination of the commercially available 2,7-dimethylnaphthalene using the procedure described by Reid and co-workers [2]. Our inital attempts of preparing 2,7-bis(fluoromethyl)naphathalene by treatment of the known 2,7-bis(hydroxymethyl)naphthalene [3] with Olah’s HF/pyridine reagent [4,5] met with failure. The alternative strategy was to employ halogen exchange using the bromo derivative 1 with a proper source of nucleophilic fluoride. The choice of fluoride source was cesium fluoride, which has appreciable solubility in polar aprotic organic solvents [6], especially when used together with a quaternary ammonium salt [6]. We would like to report a convenient procedure for preparation of 2,7-bis(fluoromethyl)naphthalene.

2. Experimental
2.1. General
All chemicals were obtained from commercial sources and were used without further purification. Melting points were determined using Mel-Temp apparatus and were uncorrected. NMR spectra were recorded on Bruker 400 MHz instrument using CDCl3 as solvent and tetramethylsilane as internal standard. The IR spectra were recorded on Perkin Elmer Model 1600 instrument between sodium chloride plates in carbon tetrachloride. The mass spectra were recorded on Kratos MS-25 RFA double focusing mass spectrometer in electron impact (EI) mode. Gas chromatography was performed on a Varian 3700 instrument with packed column. The column was 1/8’’ in diameter and 50 cm in length packed with 5% OV-101 on supelcoport and was purchased from Supelco. The carrier gas was helium (30mL/min flow) , the detection was accomplished with flame ionization and monitored with HP-3390A reporting integrator. Preparative flash chromatography [7] was performed using Merck silica gel 60 (230-400 mesh) and TLC was carried out using Merck pre-coated plates (60 F254, 250 μm).
2.2. 2,7-Bis(bromomethyl)naphthalene (1)
2,7-Bis(bromomethyl)naphthalene (1) was prepared by the procedure described by Reid et.al.
Mp 144-146 °C (lit. [2] 145-147 °C).
Rf = 0.32 (10% dichloromethane in hexane).
GC Rt = 11.87 min (100 °C, 3 min, 8 °C/min 280 °C).
1H NMR (400 MHz, CDCl3): δ = 7.83-7.79 (m, 4H), 7.51 (d, J = 8.8 Hz, 2H), 4.65 (s, 4H).
13C NMR (100 MHz, CDCl3): δ = 136.1, 133.2, 132.9, 128.8, 128.1, 127.7 (Ar), 33.9 (Ar-CH2-Br).
2.3 2,7-Bis(fluoromethyl)naphthalene (2)
A mixture of 2,7-bis(bromomethyl)naphthalene, 1, (1.258 g, 4.01 mmol), dry cesium fluoride (1.341 g, 8.82 mmol), tetraethylammonium bromide (0.050 g, 0.238 mmol) in dry acetonitrile (20 mL) was refluxed for 24 hours with vigorous stirring under argon. Additional cesium fluoride was added (1.341 g, 2.61 mmol) and the mixture was refluxed for an additional 24 hours. The reaction mixture was cooled to room temperature, diluted with methylene chloride (75 mL) and transferred to a separatory funnel. The organic layer was washed with water (5 × 50 mL), saturated sodium chloride (1 × 50 mL) and dried over sodium sulfate. The solvents were removed in vacuo and the resulting white powder was subjected to column chromatography (SiO2) eluting with 10% dichloromethane in hexane to yield 0.477 g (62%) of 2 as a white crystalline solid.
Mp 127-129 °C; Rf = 0.21 (10% methylene chloride in hexanes).
GC Rt = 4.41 min (100 °C, 3 min, 8 °C/min 280 °C).
1H NMR (400 MHz, CDCl3): δ = 7.86 (d, J = 8.4 Hz, 2H), 7.82 (broad s, 2H), 7.49 (d, J = 8.4 Hz, 2H), 5.53 (d, 2JH-F = 47.8 Hz, 4H).
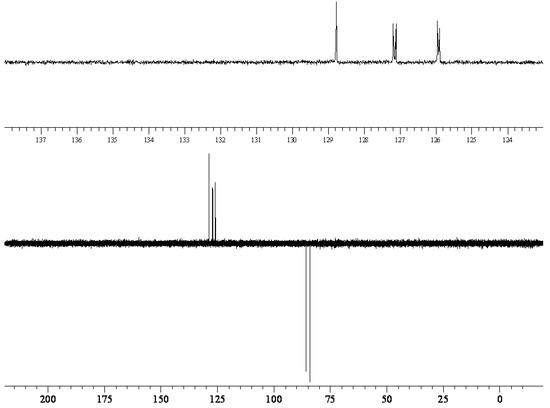
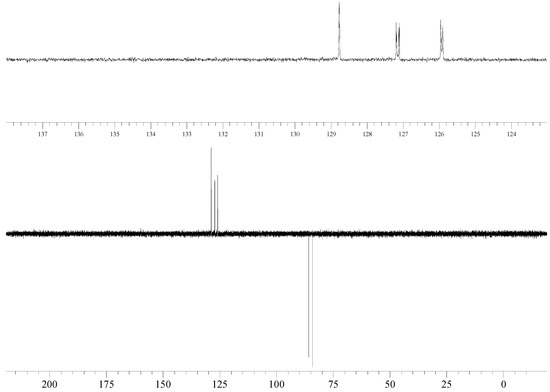
13C NMR (100 MHz, CDCl3): δ = 134.68 (d, 2JC-F = 17 Hz), 133.68, 133.26, 128.76, 127.14 (d, 3JC-F = 8 Hz, CH), 125.92 (d, 3JC-F = 6 Hz) (Ar), 84.99 (d, 1JC-F = 166 Hz, Ar-CH2-F).
135DEPT NMR: 128.76 (↑, CH), 127.14 (↑, d, 3JC-F = 8 Hz, CH), 125.92 (↑, d, 3JC-F = 6 Hz, CH), 84.99 (↓, d, 3JC-F = 6 Hz, Ar-CH2-F).
IR (CCl4, cm-1): 3060, 3033, 2955, 2894, 1614, 1518, 1465, 1439, 1381, 1362, 1340, 1272, 1205, 1172, 1032, 1014, 990, 956, 904.
EI-MS (m/z, rel. intensity): 193 (M+ + 1, 10%), 192 (M+, 78%), 191 (M+ - 1, 30%), 190 (7%), 173 (M+ - F, 6%), 172 (M+ - HF, 16%), 170 (17%), 159 (100%), 133 (13%).
3. Conclusion
The reaction of 2,7-bis(bromomethyl)naphthalene with cesium fluoride in refluxing acetonitrile, in the presence of tetraethylammonium bromide gave the desired product 2 as a white crystalline solid. The reaction can be conveniently monitored by thin-layer chromatography or gas chromatography. It is important to push the halogen exchange to completion, because the intermediate 2-bromomethyl-7-fluoromethylnaphthalene has similar polarity with 2. The structure of 2 was unambiguously determined by spectroscopic means (1H NMR, 13C NMR, 135DEPT NMR, IR and MS). The presence of the benzylic fluorine could be seen from the 1H NMR spectrum (doublet centered at 5.53 ppm, arising from two bond hydrogen-fluorine coupling, with coupling constant of 47.8 Hz) and from the 13C NMR spectrum (doublet centered at 85.0 ppm arising from one bond carbon-fluorine coupling with coupling constant 166 Hz). Both values are in excellent agreement with the literature values for benzylic fluorides [8,9].
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Maslak, P.; Narvaez, J.N. Mesolytic Cleavage of CC Bonds. Com- parison with Homolytic and Heterolytic Processes in the Same Substrate. Angew. Chem. Int. Ed. Engl. 1990, 29, 283–285. [Google Scholar] [CrossRef]
- Ried, W.; Bodem, H. Characteristic Derivatives of the di- and Trimethylnaphthalenes. Chem. Ber. 1958, 91, 1981–1982. [Google Scholar]
- Biewer, M.C.; Biehn, C.R.; Platz, M.S.; Despres, A.; Migirdicyan, E. An exceptionally Simple Method of Preparation of Biradicals. 2. Low-temperature Fluorescence Spectra and Ambient Temperature Laser-induced Fluorescence Spectra of 1,3-, 1,6-, 2,6-, and 2,7-Naphthoquinodimethane. J. Am. Chem. Soc. 1991, 113, 616–620. [Google Scholar] [CrossRef]
- Olah, G.A.; Nojima, M.; Kerekes, I. Synthetic Methods and Reactions II1. Hydrofluorination of Alkenes, Cyclopropane and Alkynes with Poly-Hydrogen Fluoride/Pyridine (Trialkylamine) Reagents. Synthesis 1973, 779–780. [Google Scholar] [CrossRef]
- Olah, G.A.; Nojima, M.; Kerekes, I. Synthetic Methods and Reactions IX. Fluorination of Secondary- and Tertiary-Alcohols with Polyhydrogen Fluoride/Pyridine(Trialkylamine) Reagents. Synthesis 1973, 786–787. [Google Scholar] [CrossRef]
- Clark, J.H. Fluoride Ion as a Base in Organic Synthesis. Chem. Rev. 1980, 80, 429–452. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 5th ed.; Wiley: New York, 1991; p. 281. [Google Scholar]
- Adcock, W.; Abeywickrema, A.N. Conformational preference of the fluoromethyl group in some benzyl fluorides: a 13C N.M.R. study. Aust. J. Chem. 1980, 33, 181–187. [Google Scholar] [CrossRef]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).