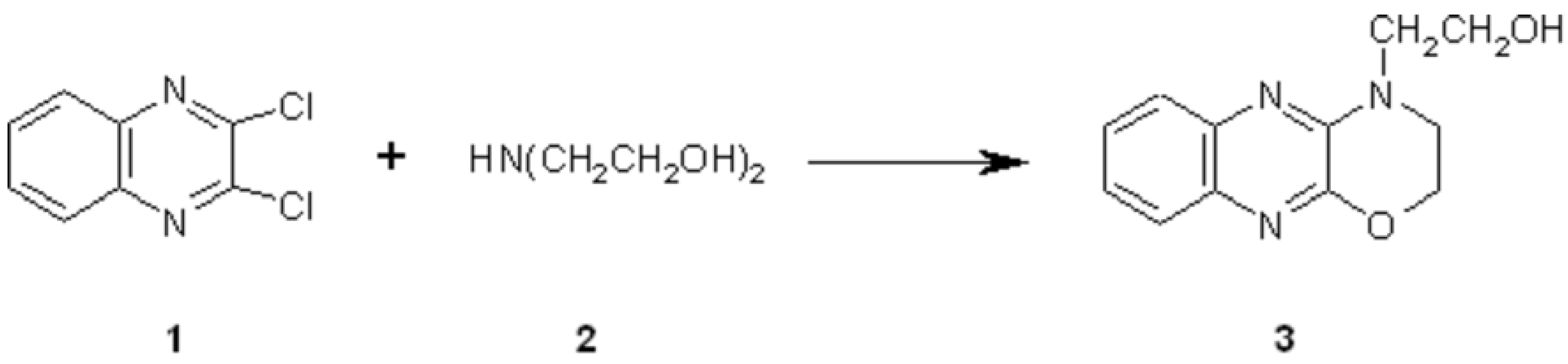
Synthesis of 2-(1,4-oxazino[2,3-b]quinoxalin-4-yl)ethanol
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Obafemi, C.A.; Pfleiderer, Wolfgang. Helv. Chim. Acta 1994, 77, 1549–1556. [CrossRef]
- Goncharova, I.N.; Postovskii, I. Ya. J. J. Gen. Chem. USSR 1962, 32, 3271–3278, 1H-NMR, 13C-NMR and mass spectra data are not given in this paper.
© 2006 MDPI. All rights reserved.
Share and Cite
Obafemi, C.A.; Pfleiderer, W.; Taiwo, F. Synthesis of 2-(1,4-oxazino[2,3-b]quinoxalin-4-yl)ethanol. Molbank 2006, 2006, M508. https://doi.org/10.3390/M508
Obafemi CA, Pfleiderer W, Taiwo F. Synthesis of 2-(1,4-oxazino[2,3-b]quinoxalin-4-yl)ethanol. Molbank. 2006; 2006(6):M508. https://doi.org/10.3390/M508
Chicago/Turabian StyleObafemi, Craig A., Wolfgang Pfleiderer, and Festus Taiwo. 2006. "Synthesis of 2-(1,4-oxazino[2,3-b]quinoxalin-4-yl)ethanol" Molbank 2006, no. 6: M508. https://doi.org/10.3390/M508
APA StyleObafemi, C. A., Pfleiderer, W., & Taiwo, F. (2006). Synthesis of 2-(1,4-oxazino[2,3-b]quinoxalin-4-yl)ethanol. Molbank, 2006(6), M508. https://doi.org/10.3390/M508



