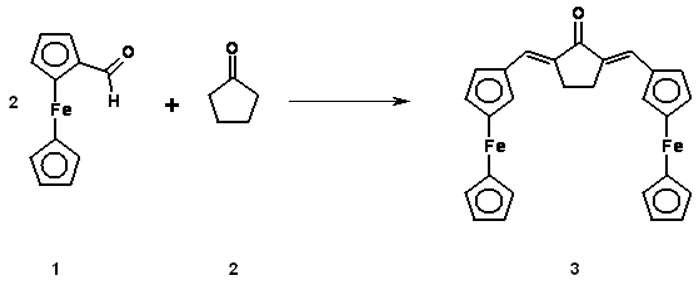
2,5-Diferrocenylidenecyclopentanone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Boyd, R.W. Nonlinear Optics; Academic Press: New York, 1992. [Google Scholar]
- Marder, S.R.; Beratan, D.N.; Cheng, L.T. Science 1991, 252, 103.
- Eaton, D.F. Science 1991, 253, 281.
- Staring, E.G. Recl. Trav. Chim. Pays-Bas 1991, 110, 492.
- Derik, C.W.; Wagniere, R.J. J. Am. Chem. Soc. 1986, 108, 5387.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Asiri, A.M. 2,5-Diferrocenylidenecyclopentanone. Molbank 2005, 2005, M412. https://doi.org/10.3390/M412
Asiri AM. 2,5-Diferrocenylidenecyclopentanone. Molbank. 2005; 2005(2):M412. https://doi.org/10.3390/M412
Chicago/Turabian StyleAsiri, Abdullah Mohamed. 2005. "2,5-Diferrocenylidenecyclopentanone" Molbank 2005, no. 2: M412. https://doi.org/10.3390/M412
APA StyleAsiri, A. M. (2005). 2,5-Diferrocenylidenecyclopentanone. Molbank, 2005(2), M412. https://doi.org/10.3390/M412



