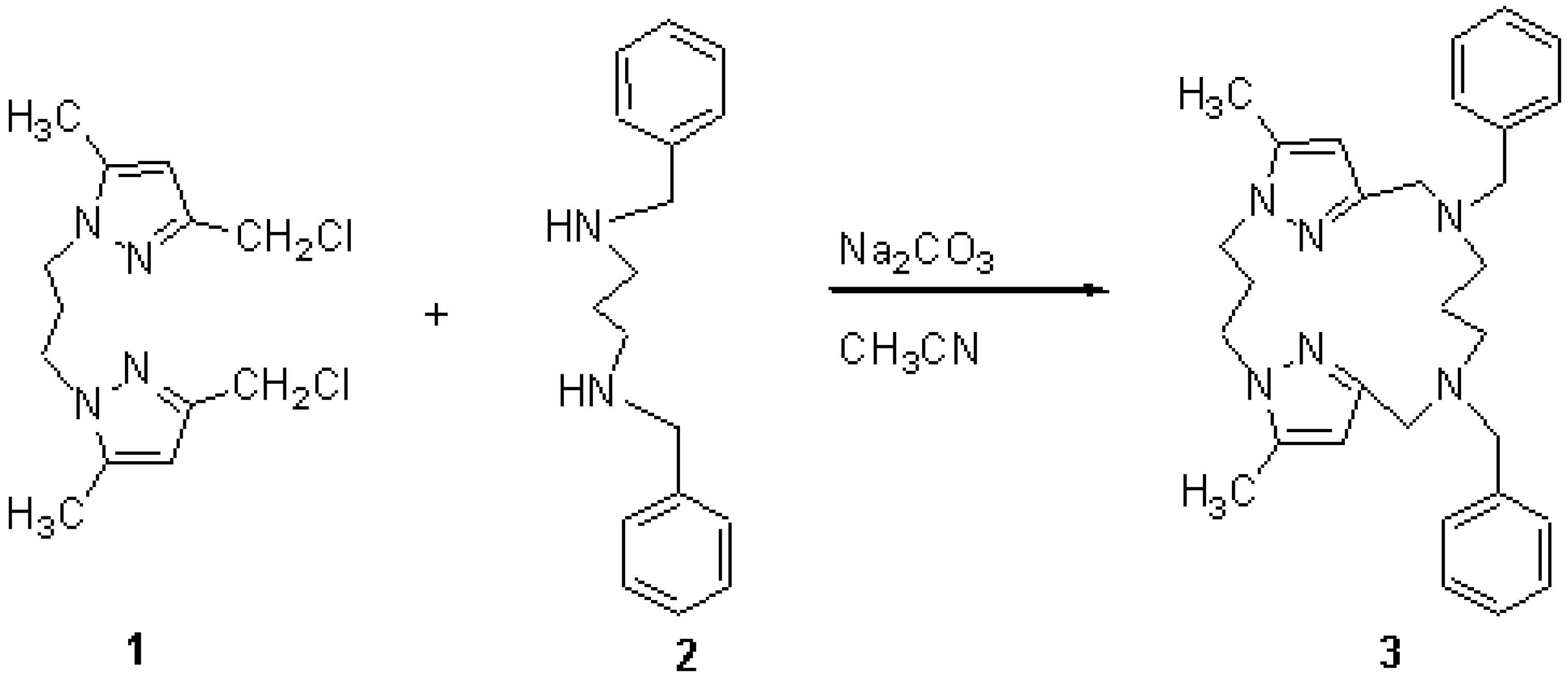
10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaazatricyclo[14.2.1.15,8]icosa-6,8(20), 16(19), 17-tetraene
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Tarrago, G.; El Kadiri, S.; Marzin, C.; Coquelet, C. New J. Chem. 1991, 15, 677.
- Bienvenue, E.; Choua, S.; Lobo-Recio, M-Has.; Marzin, C.; Pacheco, P.; Seta, P.; Tarrago, G. Inorg. Biochem. 1995, 57, 157.
- Bol, J. E.; Driessen, W. L.; Reedjik, J. J. Chem. Soc., Chem. Commun 1995, 13, 65.
© 2003 MDPI. All rights reserved.
Share and Cite
Berhili, F.; Touzani, R.; Ramdani, A.; El Kadiri, S. 10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaazatricyclo[14.2.1.15,8]icosa-6,8(20), 16(19), 17-tetraene. Molbank 2003, 2003, M312. https://doi.org/10.3390/M312
Berhili F, Touzani R, Ramdani A, El Kadiri S. 10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaazatricyclo[14.2.1.15,8]icosa-6,8(20), 16(19), 17-tetraene. Molbank. 2003; 2003(1):M312. https://doi.org/10.3390/M312
Chicago/Turabian StyleBerhili, Farid, Rachid Touzani, Abdelkrim Ramdani, and Sghir El Kadiri. 2003. "10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaazatricyclo[14.2.1.15,8]icosa-6,8(20), 16(19), 17-tetraene" Molbank 2003, no. 1: M312. https://doi.org/10.3390/M312
APA StyleBerhili, F., Touzani, R., Ramdani, A., & El Kadiri, S. (2003). 10,14-Dibenzyl-6,18-dimethyl-1,5,10,14,19,20-hexaazatricyclo[14.2.1.15,8]icosa-6,8(20), 16(19), 17-tetraene. Molbank, 2003(1), M312. https://doi.org/10.3390/M312



