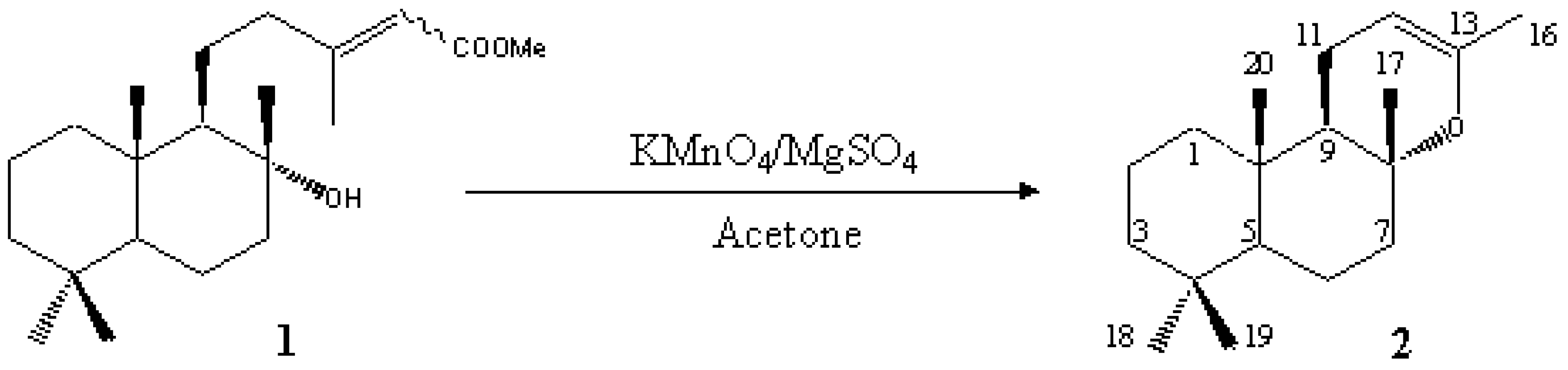
8a,13-Epoxy-14,15-dinorlabd-12-ene (Sclareol Oxide) [(+)-(4aR,6aS,10aS,10bR)-3,4a,7,7,10a-Pentamethyl-4a,5,6,6a,7,8,9,10,10a,10b-decahydro-1H-benzo[f]chromene]
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Leite, M. A. F.; Sarragiotto, M. H.; Imamura, P. M.; Marsaioli, A. J. Absolute Configuration of Drim-9(11)-en-8-ol from Aspergillus oryzae. J. Org. Chem. 1986, 51, 5409–5410. [Google Scholar] [CrossRef]
- Sample availability: Available from the authors and from MDPI
© 2003 MDPI. All rights reserved.
Share and Cite
Castro, J.M.; Salido, S.; Altarejos, J.; Nogueras, M.; Sanchez, A. 8a,13-Epoxy-14,15-dinorlabd-12-ene (Sclareol Oxide) [(+)-(4aR,6aS,10aS,10bR)-3,4a,7,7,10a-Pentamethyl-4a,5,6,6a,7,8,9,10,10a,10b-decahydro-1H-benzo[f]chromene]. Molbank 2003, 2003, M304. https://doi.org/10.3390/M304
Castro JM, Salido S, Altarejos J, Nogueras M, Sanchez A. 8a,13-Epoxy-14,15-dinorlabd-12-ene (Sclareol Oxide) [(+)-(4aR,6aS,10aS,10bR)-3,4a,7,7,10a-Pentamethyl-4a,5,6,6a,7,8,9,10,10a,10b-decahydro-1H-benzo[f]chromene]. Molbank. 2003; 2003(1):M304. https://doi.org/10.3390/M304
Chicago/Turabian StyleCastro, Juan M., Sofia Salido, Joaquin Altarejos, Manuel Nogueras, and Adolfo Sanchez. 2003. "8a,13-Epoxy-14,15-dinorlabd-12-ene (Sclareol Oxide) [(+)-(4aR,6aS,10aS,10bR)-3,4a,7,7,10a-Pentamethyl-4a,5,6,6a,7,8,9,10,10a,10b-decahydro-1H-benzo[f]chromene]" Molbank 2003, no. 1: M304. https://doi.org/10.3390/M304
APA StyleCastro, J. M., Salido, S., Altarejos, J., Nogueras, M., & Sanchez, A. (2003). 8a,13-Epoxy-14,15-dinorlabd-12-ene (Sclareol Oxide) [(+)-(4aR,6aS,10aS,10bR)-3,4a,7,7,10a-Pentamethyl-4a,5,6,6a,7,8,9,10,10a,10b-decahydro-1H-benzo[f]chromene]. Molbank, 2003(1), M304. https://doi.org/10.3390/M304



