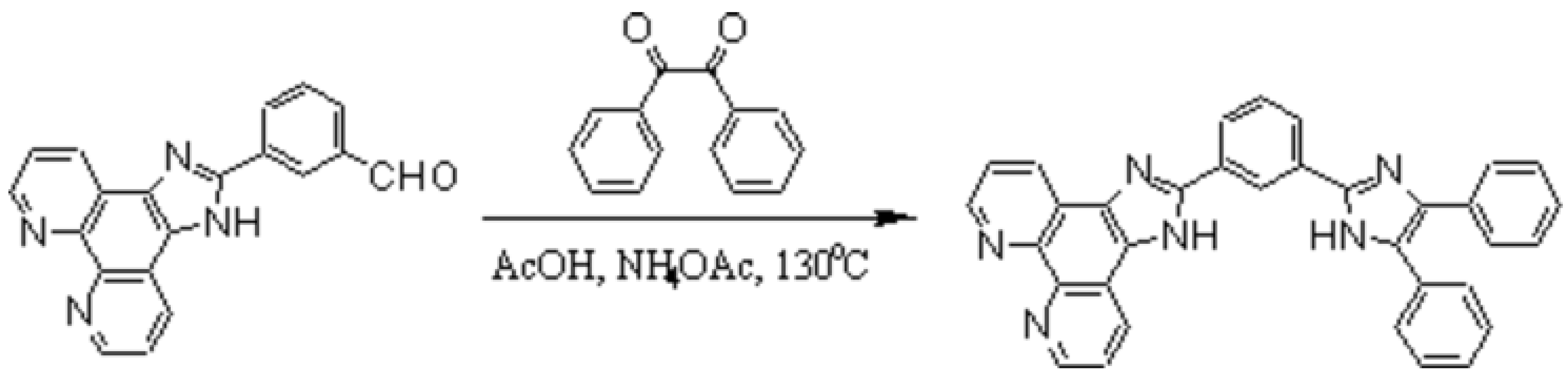
(1-(Phenanthrolo[5,6-d]imidazol-2-yl)-3-(4,5-diphenylimidazol-2-yl)benzene
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References and Notes
- Chao, H.; Ye, B.-H.; Li, H.; Li, R.-H.; Zhou, J.-Y.; Ji, L.-N. Polyhedron 2000, 19, 1975.
- Sample availabylitiy: available from the authors
© 2003 MDPI. All rights reserved.
Share and Cite
Chao, H.; Jiang, C.-W.; Zhang, H.; Ji, L.-N. (1-(Phenanthrolo[5,6-d]imidazol-2-yl)-3-(4,5-diphenylimidazol-2-yl)benzene. Molbank 2002, 2002, M282. https://doi.org/10.3390/M282
Chao H, Jiang C-W, Zhang H, Ji L-N. (1-(Phenanthrolo[5,6-d]imidazol-2-yl)-3-(4,5-diphenylimidazol-2-yl)benzene. Molbank. 2002; 2002(1):M282. https://doi.org/10.3390/M282
Chicago/Turabian StyleChao, Hui, Cai-Wu Jiang, Hao Zhang, and Liang-Nian Ji. 2002. "(1-(Phenanthrolo[5,6-d]imidazol-2-yl)-3-(4,5-diphenylimidazol-2-yl)benzene" Molbank 2002, no. 1: M282. https://doi.org/10.3390/M282
APA StyleChao, H., Jiang, C.-W., Zhang, H., & Ji, L.-N. (2002). (1-(Phenanthrolo[5,6-d]imidazol-2-yl)-3-(4,5-diphenylimidazol-2-yl)benzene. Molbank, 2002(1), M282. https://doi.org/10.3390/M282



