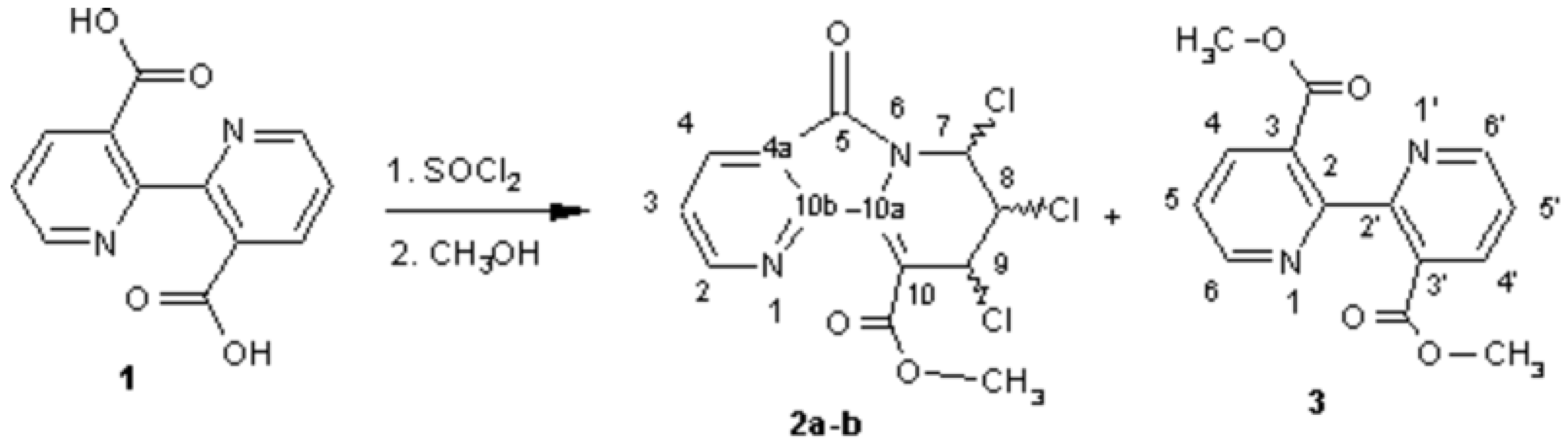
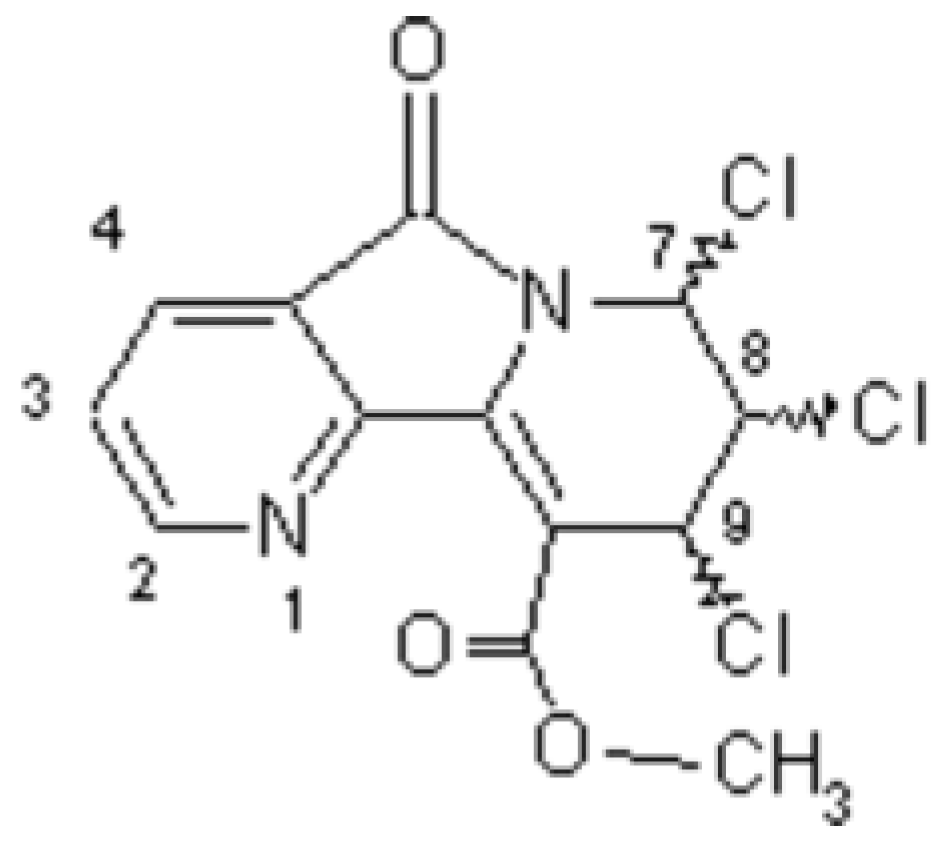
Chiral Methyl 7,8,9-Trichloro-6,7,8,9-tetrahydro-5-oxopyrido-[2,3-a]indolizine-10-carboxylates (OPIC)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgement
References and Notes
- Blau, F. Ber. Dtsch. Chem. Ges. 1888, 21, 1077.
- Ben-Hadda, T.; Sam, N.; Le Bozec, H.; Dixneuf, P. H. Inorg. Chem. Com 1999, 2, 460.
- Sample Availability: Available from the authors
© 2003 MDPI. All rights reserved.
Share and Cite
Larbi, N.B.; Daoudi, M.; Sam, N.; Mimouni, M.; Elkadiri, S.; Ben-Hadda, T. Chiral Methyl 7,8,9-Trichloro-6,7,8,9-tetrahydro-5-oxopyrido-[2,3-a]indolizine-10-carboxylates (OPIC). Molbank 2002, 2002, M277. https://doi.org/10.3390/M277
Larbi NB, Daoudi M, Sam N, Mimouni M, Elkadiri S, Ben-Hadda T. Chiral Methyl 7,8,9-Trichloro-6,7,8,9-tetrahydro-5-oxopyrido-[2,3-a]indolizine-10-carboxylates (OPIC). Molbank. 2002; 2002(1):M277. https://doi.org/10.3390/M277
Chicago/Turabian StyleLarbi, Najib Ben, Maria Daoudi, Najat Sam, Mostafa Mimouni, Sghir Elkadiri, and Taibi Ben-Hadda. 2002. "Chiral Methyl 7,8,9-Trichloro-6,7,8,9-tetrahydro-5-oxopyrido-[2,3-a]indolizine-10-carboxylates (OPIC)" Molbank 2002, no. 1: M277. https://doi.org/10.3390/M277
APA StyleLarbi, N. B., Daoudi, M., Sam, N., Mimouni, M., Elkadiri, S., & Ben-Hadda, T. (2002). Chiral Methyl 7,8,9-Trichloro-6,7,8,9-tetrahydro-5-oxopyrido-[2,3-a]indolizine-10-carboxylates (OPIC). Molbank, 2002(1), M277. https://doi.org/10.3390/M277



