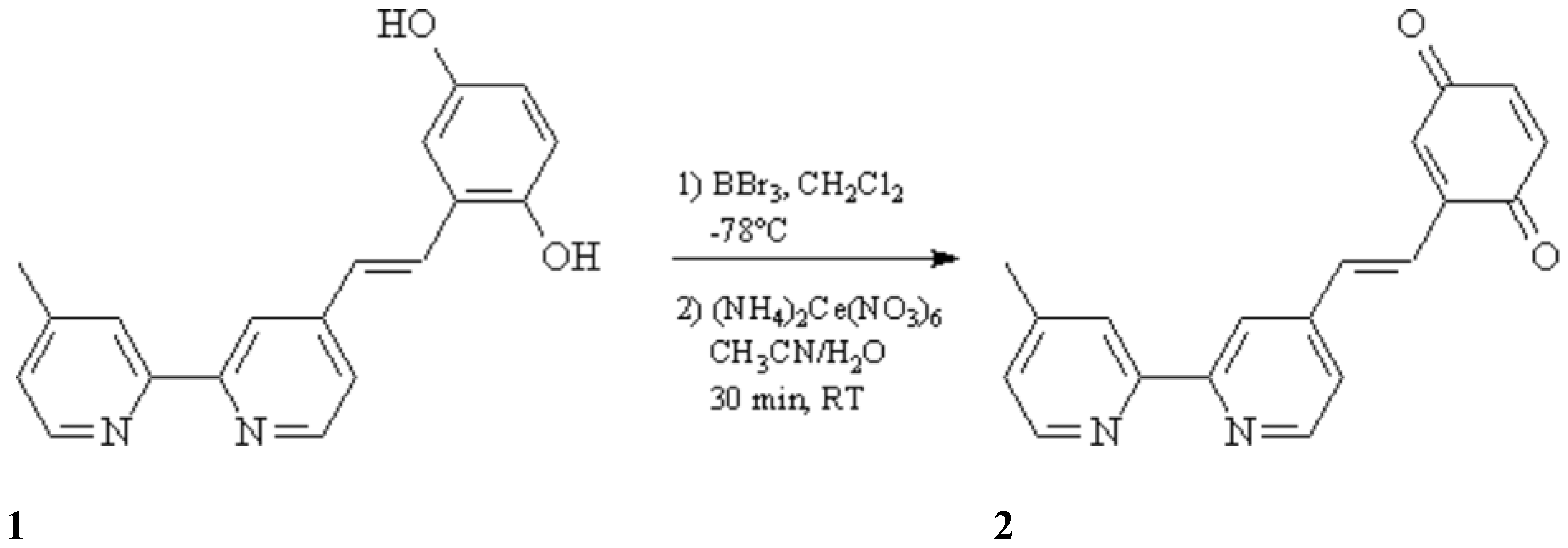
Lehn et al. [
1] have reported the synthesis of a compound similar to
2, which has a saturated ethano bridged between the 2,2'-bipyridine and the
para-benzoquinone moiety. We report here the synthesis of the unsaturated compound
2, starting from the same starting material
1 [
1] and using standard BBr
3 procedures [
2] for the cleavage of the methyl ether. The intermediate obtained hydroquinone is oxidized to the target quinone using cerium(IV)ammonium nitrate [
3] as oxidant.
To a solution of 4-(2,5-dimethoxystyryl)-4'-methyl-2,2'-bipyridine (1.58 g, 4.8 mmol) (1) in dry dichloromethane (100 mL) were added at -78 °C 3.7 mL of boron tribromide. The reaction mixture was kept overnight at room temperature, poured into 150 mL of ice water and adjusted to neutral pH with aqueous sodium hydroxide (1.5 M), whereby the hydroquinone precipitates. The precipitate was filtered off, washed with methanol and ether, and suspended in 80 mL of a mixture of acetonitrile / water (3:1). Cerium(IV)ammonium nitrate (3.72 g, 6.8 mmol) was added, the suspension was stirred at room temperature for 30 min, adjusted to neutral pH by addition of saturated NaHCO3 solution and filtered. The precipitate was washed with dichloromethane, the combined organic phases were dried over MgSO4 and evaporated in vacuo to dryness. The crude product was purified by column chromatography on silica gel [CH2Cl2/MeOH/NH3 (25% in water) 100:5:0.5] to yield 180 mg (13%) of 2 (Rf = 0.5); yellow-brownish solid, mp. 151 °C.
IR (KBr, cm-1) = 1656, 1591, 1463, 1298, 1069, 981, 814.
UV/Vis (CH3CN):max (log e) = 204 nm (4.512), 248 (4.253), 284 (4.308), 332 (3.991).
1H NMR (400 MHz, CDCl3): d = 2.45 (s, 3H, bipyridine-CH3), 6.81 (m, 2H, quinone -H), 6.90 (m, 1H, quinone-H), 7.17 (d, 1H, 3J = 4.8 Hz, bipyridine-H), 7.35 (d,3J = 16.5 Hz, 1H, vinyl-H), 7.40 (d, 1H, 3J = 5.1 Hz, bipyridine-H), 7.50 (d, 1H, 3J = 16.5 Hz, vinyl-H), 8.25 (s, 1H, bipyridine-H), 8.51 (s, 1H, bipyridine-H), 8.56 (d, 3J =4.9 Hz, 1H bipyridine-H), 8.67 (d, 3J = 5.3 Hz, 1H bipyridine-H).
13C NMR (100 MHz, CDCl3): d = 21.23 (+), 119.01 (+), 121.36 (+), 122.14 (+), 124.20 (+), 125.07 (+), 129.96 (+), 135.70 (+), 136.75 (+), 136.76 (+), 141.07 (Cquat), 144.17 (Cquat), 148.37 (Cquat), 149.03 (+), 149.80 (+), 155.45 (Cquat), 157.11 (Cquat), 186.49 (Cquat), 187.44 (Cquat).
MS (70 eV), m/z (%): 302 (100) [M+], 169 (48) [M+ - C8H5O2].