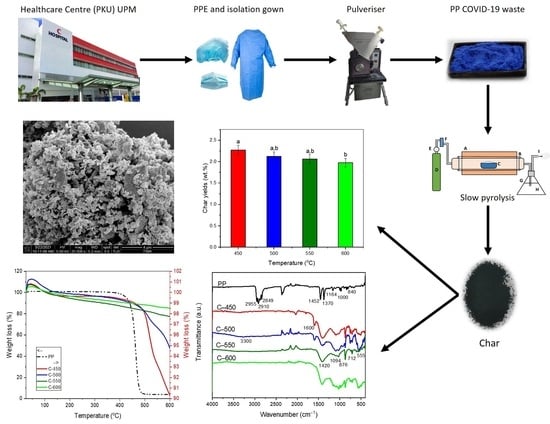
Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polypropylene (PP) Isolation Gown Waste
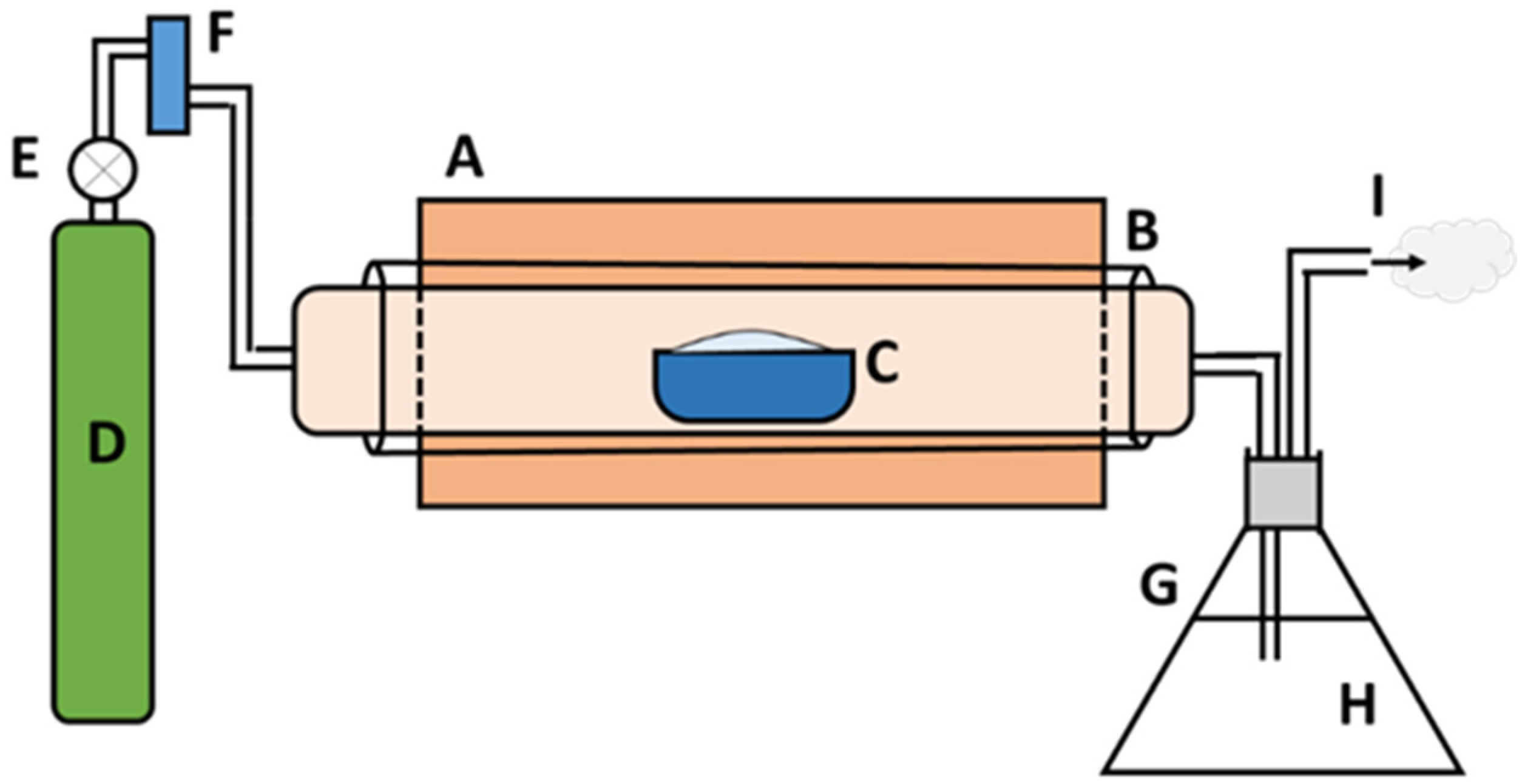
2.2. Preparation of Char
2.3. Characterisation of Char
2.3.1. Proximate Analysis
2.3.2. FESEM
2.3.3. EDX
2.3.4. FTIR
2.3.5. Raman Spectroscopy
2.3.6. BET
2.3.7. TGA-DTG
2.4. Statistical Analysis
3. Results and Discussions
3.1. Proximate Analysis of PP-IG Samples as Feedstock
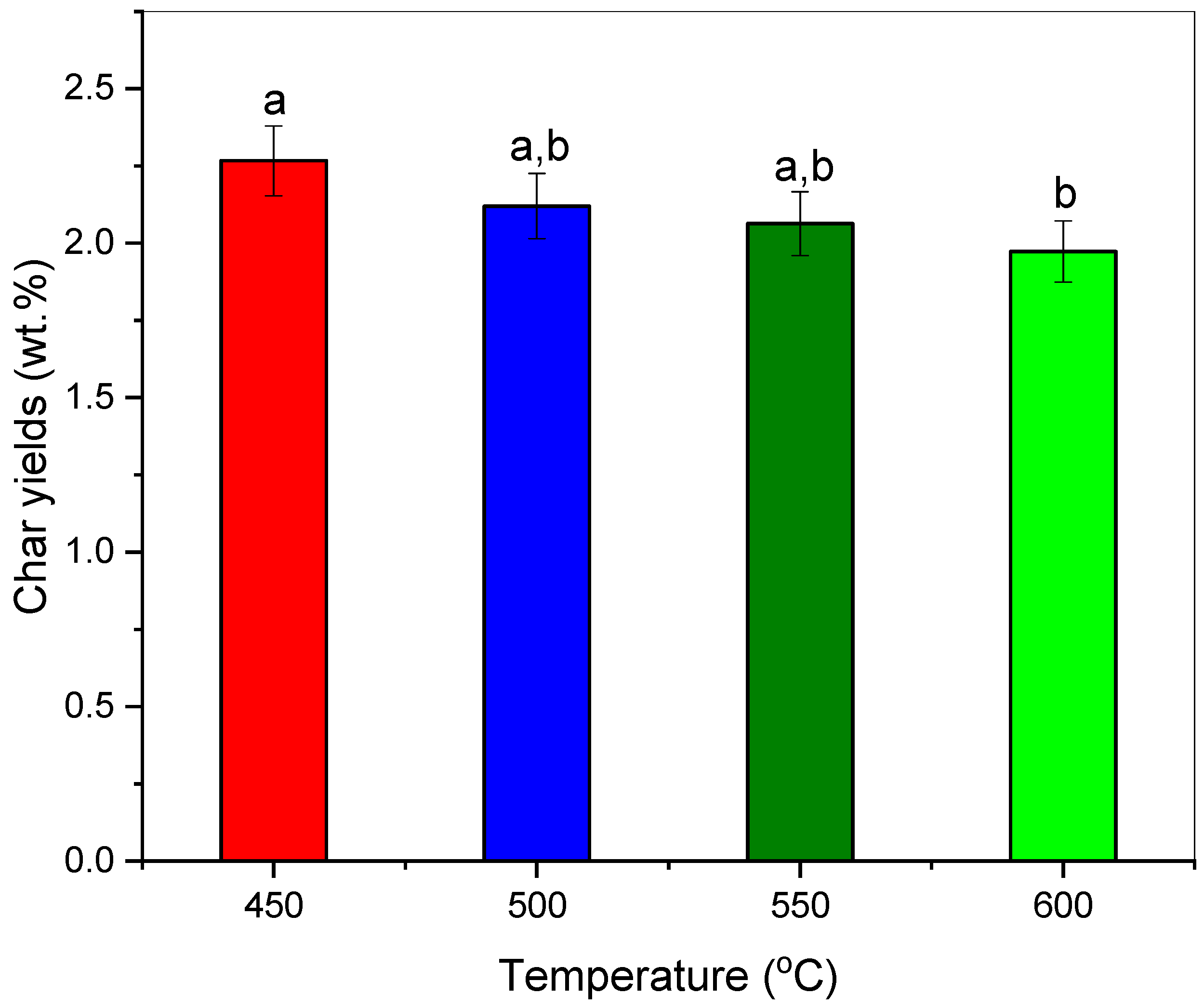
3.2. Effect of Pyrolysis Temperature towards Char Yields and Its Thermochemical Performance
3.3. Characterisation of Solid Product Char and PP-IG Samples
3.3.1. Volatile Matter Content
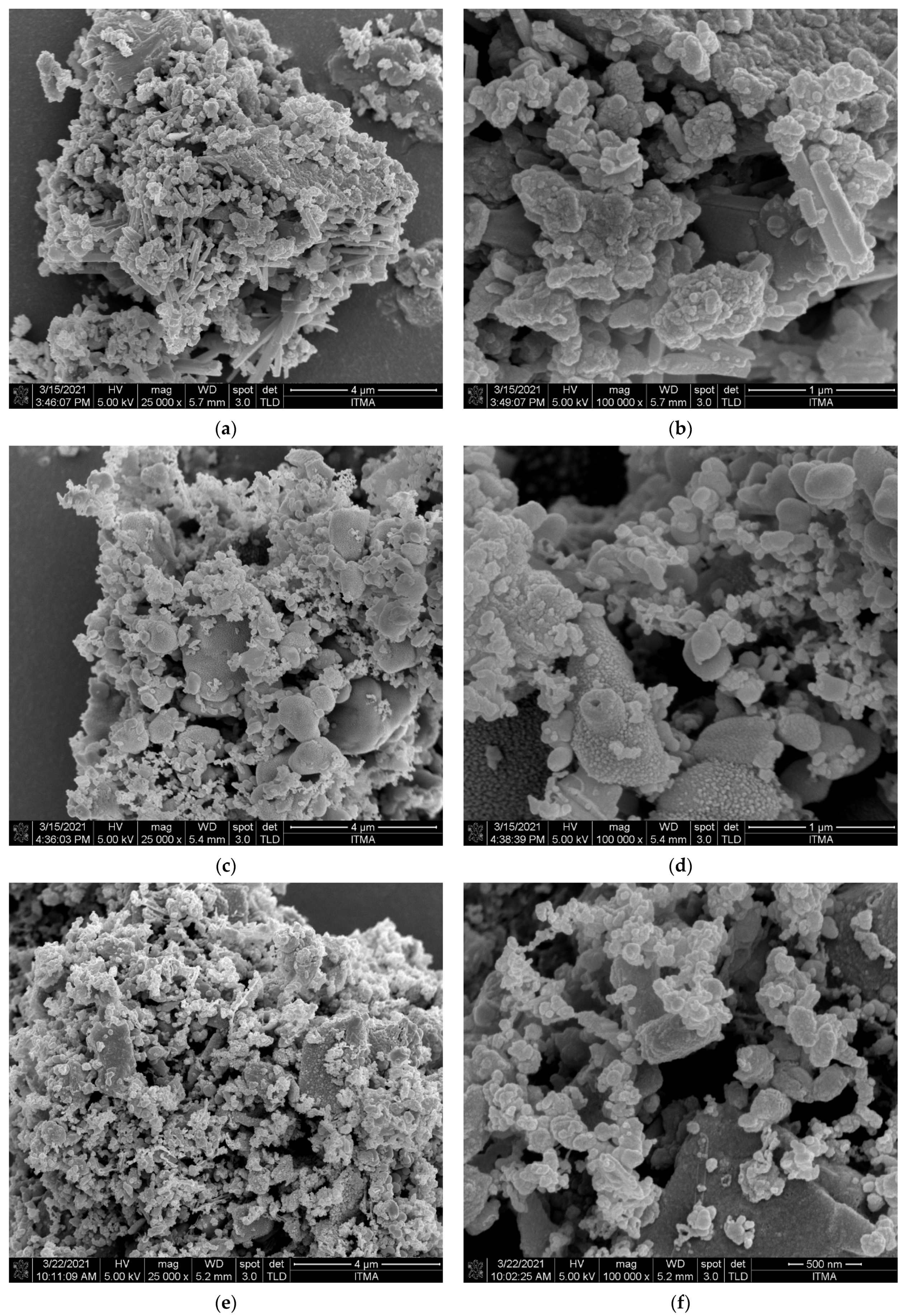
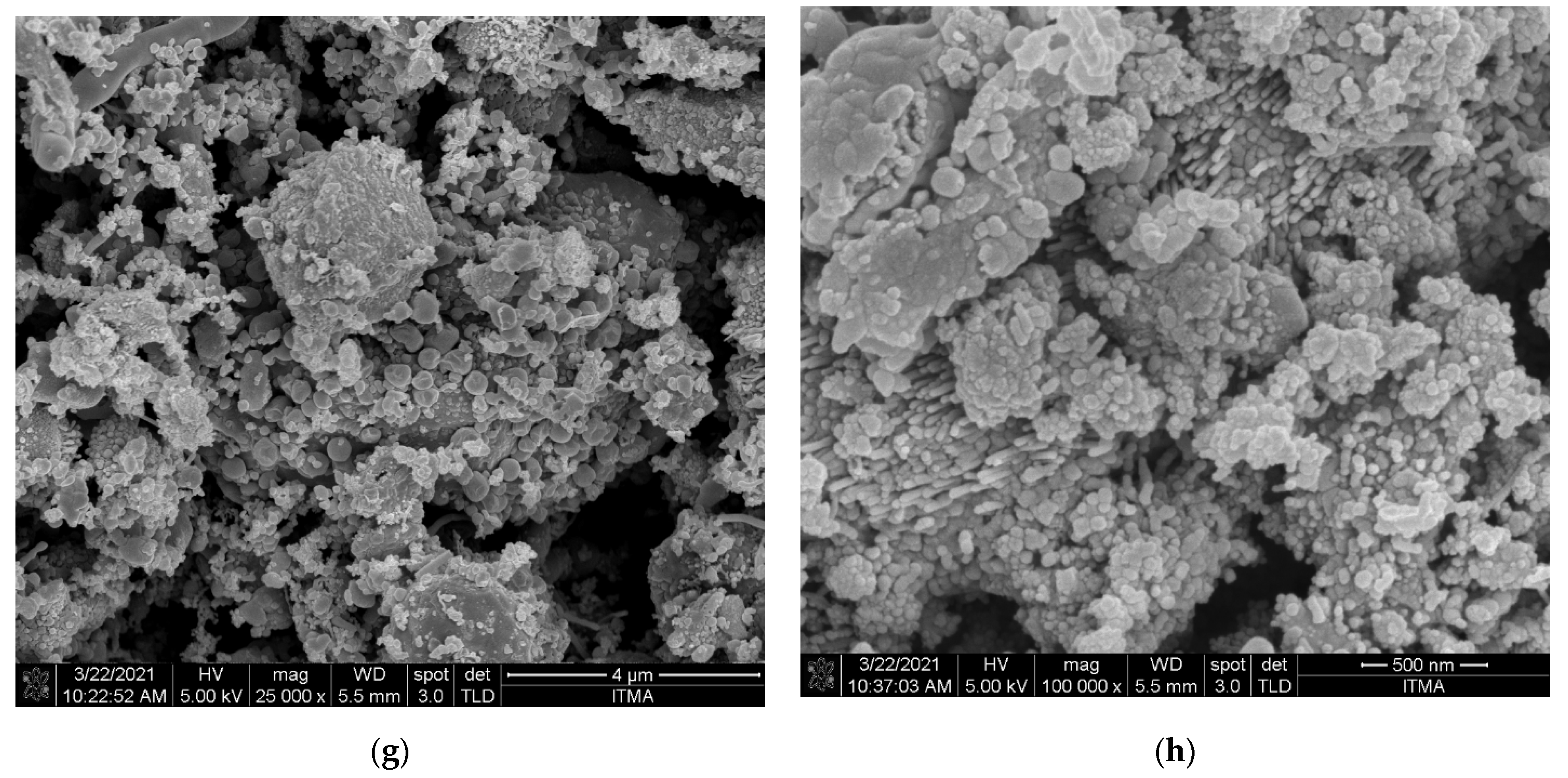
3.3.2. Surface Morphology Analysis
3.3.3. Surface Area and Porosity Measurement
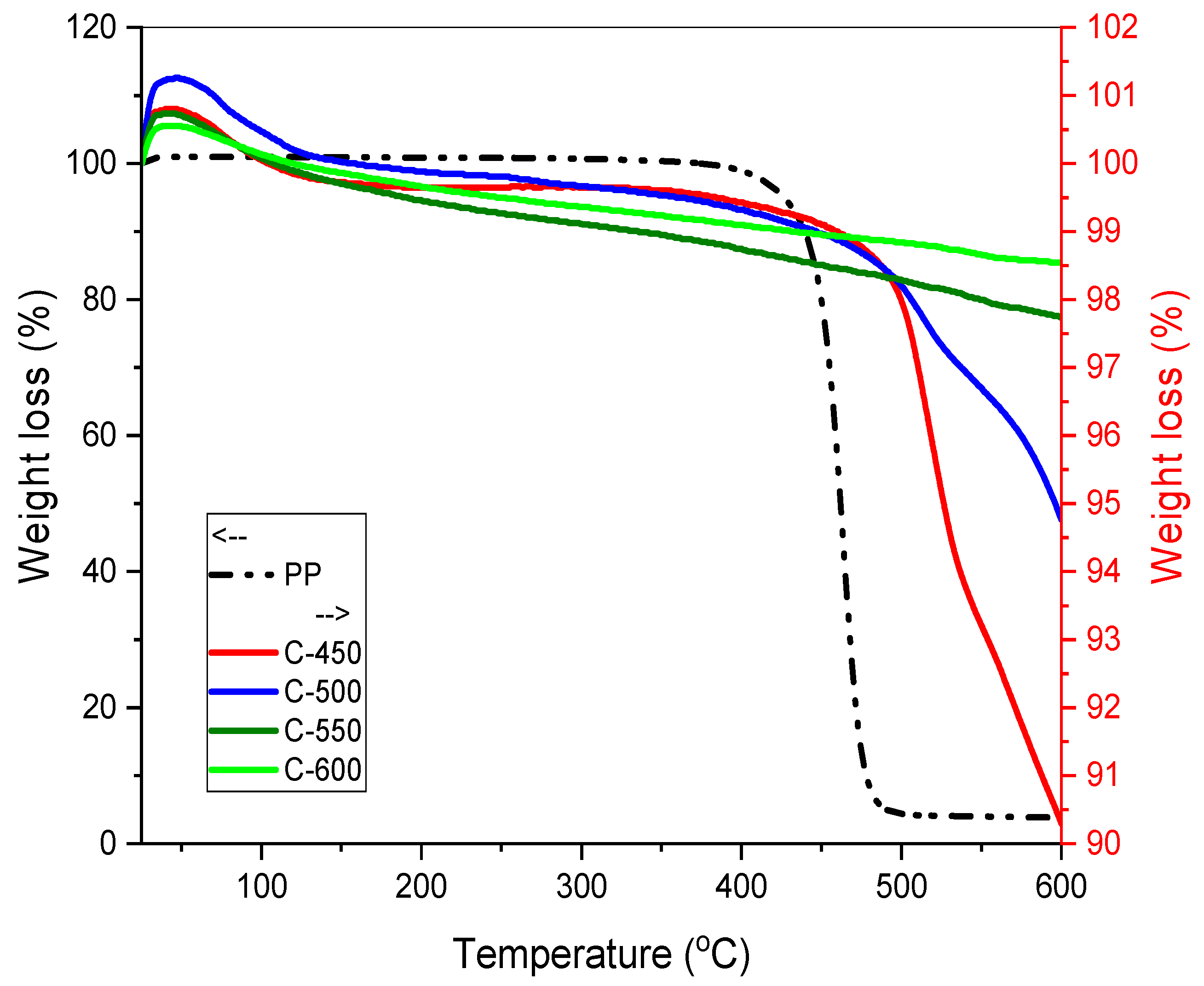
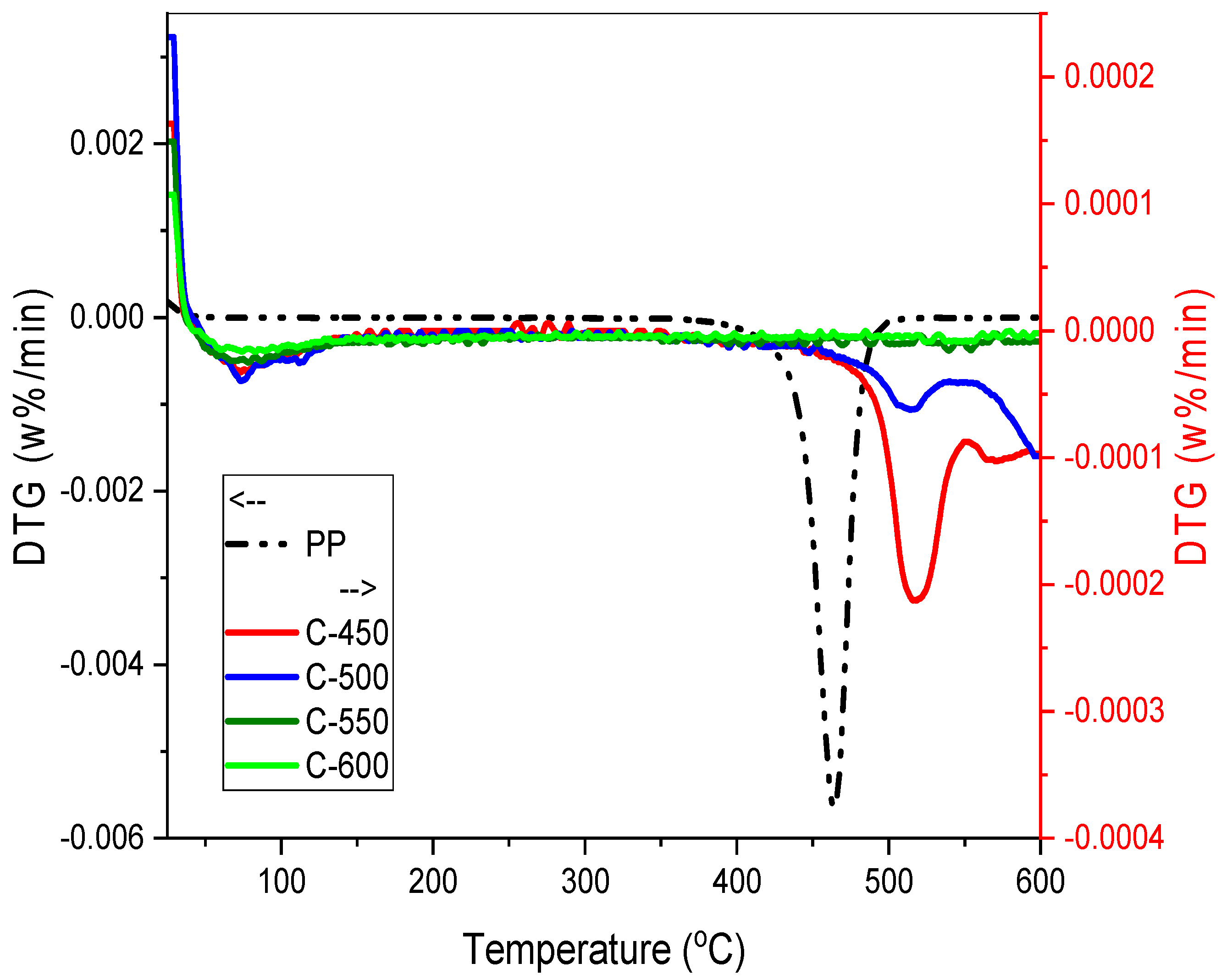
3.3.4. Thermal Gravimetric Analysis (TGA)
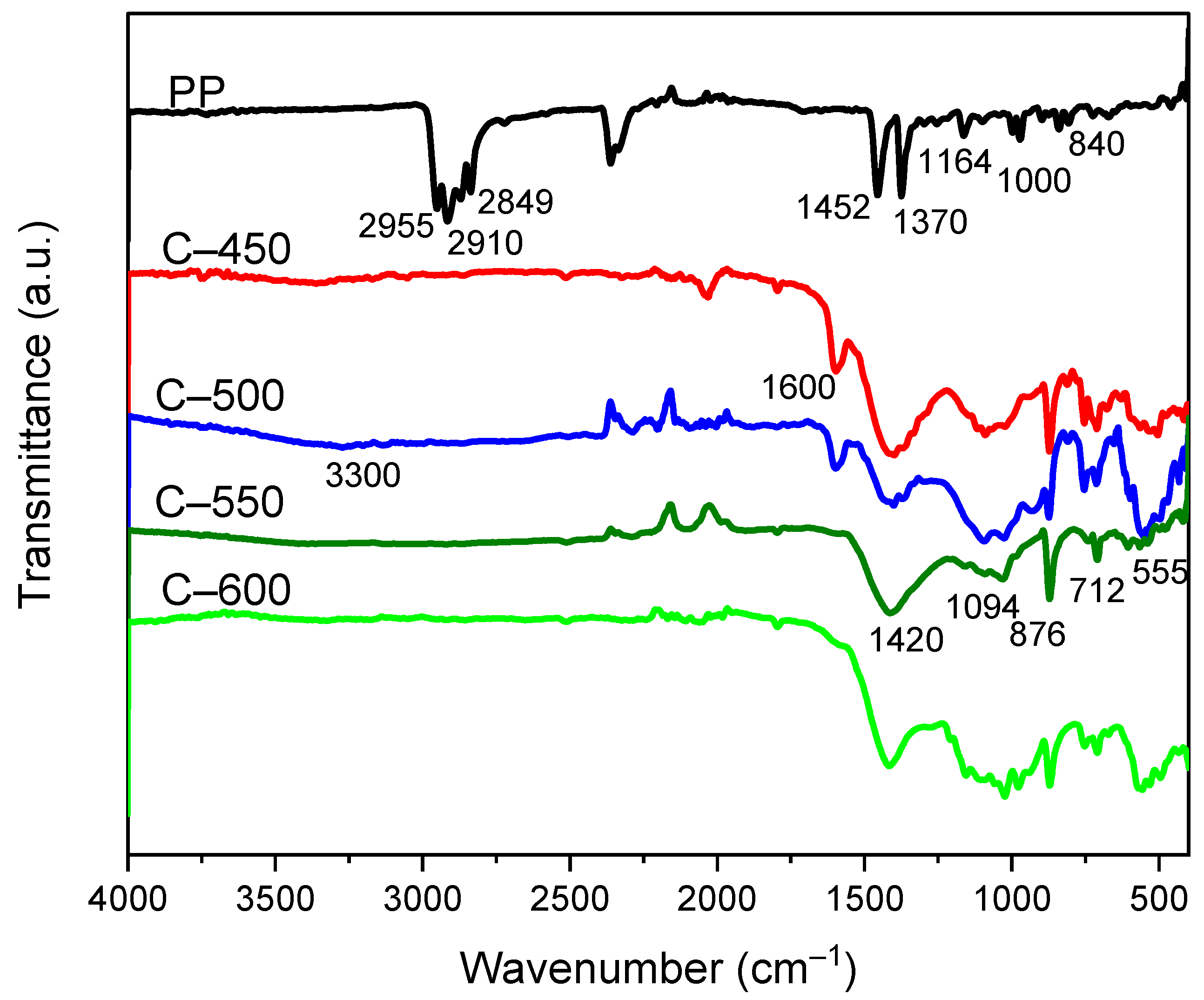
3.3.5. Functional Group Analysis
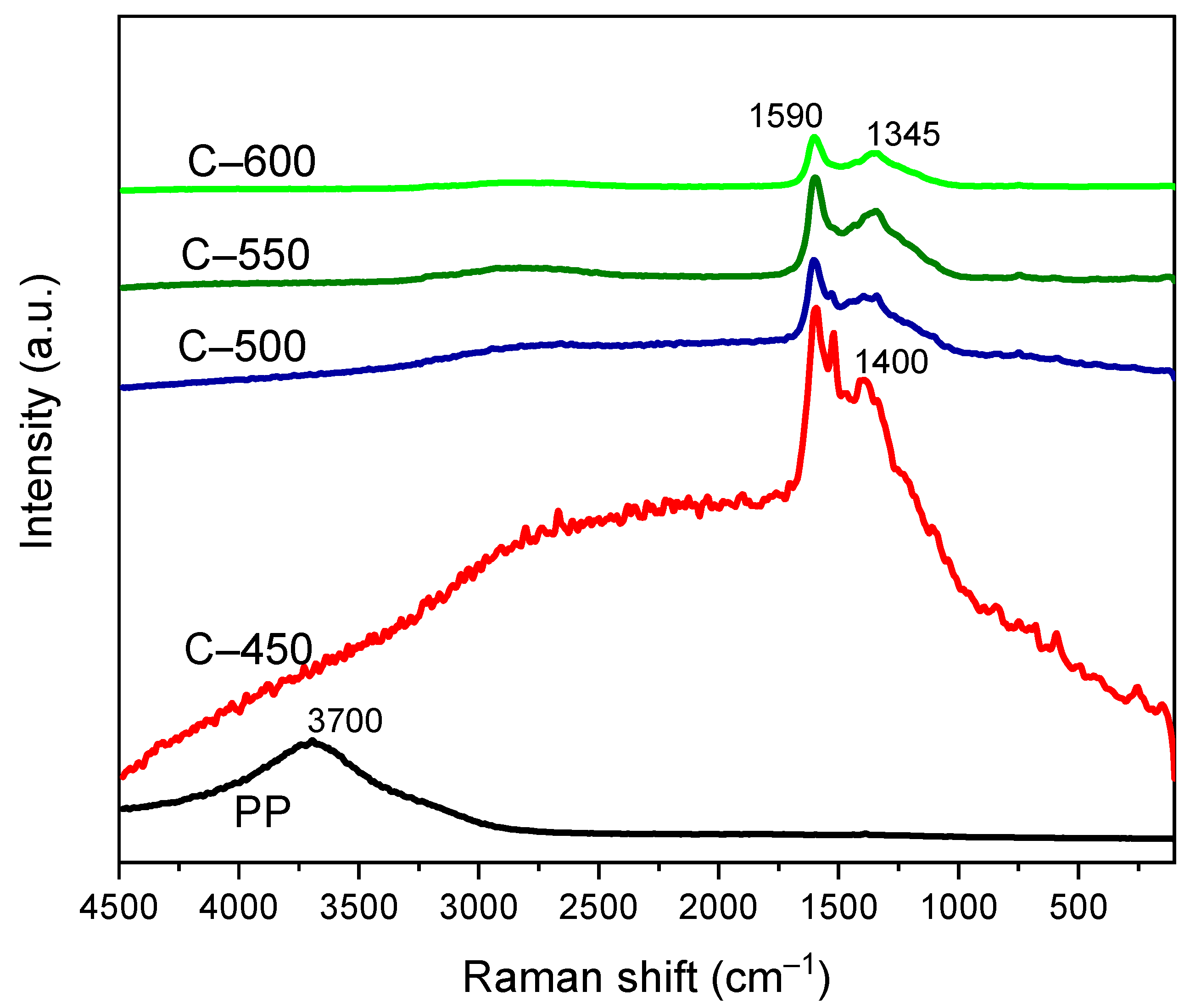
3.3.6. Raman Spectroscopy
4. Conclusions and Future Outlooks
- (i)
- The low–temperature pyrolysis of polypropylene-based COVID-19 isolation gown waste (PP-IG) resulted in improved char yields (2.27 wt.%), which mostly comprises aliphatic and carbonaceous alkene structures.
- (ii)
- On the other hand, increasing the temperature decreased the yield of char products but produced char with higher surface area value and enhanced pore volumes, ~24 m2g−1 and ~0.08 cm3g−1, respectively.
- (iii)
- The char obtained at higher temperatures (C-550 and C-600) comprised more aromatics C=C and carbonaceous C–H structures due to volatilisation and carbonisation.
- (iv)
- The low temperature (450 °C) and long residence time (5 h) degradation process supported the reaction from the polymer scission and led to a lighter hydrocarbon with reduced microporosity due to lower carbon structure deterioration.
- (v)
- In order to generate unique char products from PP-IG waste, data collected during this study are valuable for the use of pyrolysis. At the same time, further studies are needed in order to optimise the pyrolysis parameters, design, and catalyst support for better pyrolysis outputs.
- (vi)
- In addition, the isothermal process used less energy and not an isothermal process, making the present work more reliable and practical in terms of transforming COVID-19 related plastic waste into energy.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO COVID-19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 7 February 2021).
- Worldometer COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 24 February 2021).
- Purnomo, C.W.; Kurniawan, W.; Aziz, M. Technological review on thermochemical conversion of COVID-19-related medical wastes. Resour. Conserv. Recycl. 2021, 167, 105429. [Google Scholar] [CrossRef]
- Tiseo, I. Plastic Waste Worldwide—Statistics & Facts. Available online: https://www.statista.com/topics/5401/global-plastic-waste/ (accessed on 24 February 2021).
- Harussani, M.M.; Sapuan, S.M.; Khalina, A.; Ilyas, R.A.; Hazrol, M.D. Review on Green Technology Pyrolysis for Plastic Wastes. In Proceedings of the 7th Postgraduate Seminar on Natural Fibre Reinforced Polymer Composites 2020, Selangor, Malaysia, 17 November 2020; pp. 50–53. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Tang, Y.; Ogunseitan, O.A. Environmentally sustainable management of used personal protective equipment. Environ. Sci. Technol. 2020, 54, 8500–8502. [Google Scholar] [CrossRef] [PubMed]
- 3M Personal Safety Division Technical Specification Sheet: 3MTM Particulate Respirator 8210, N95. 2018. Available online: http://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/Annex_8__IPC_21032020.pdf (accessed on 24 February 2021).
- MOH. The Infection Prevention And Control (IPC) measures in managing Patient Under Investigation (PUI) or confirmed corona virus disease (COVID-19). Minist. Heal. Malaysia 2020. Available online: http://medicalprac.moh.gov.my/v2/uploads/Covid19/1.%20The%20IPC%20Measures%20for%20COVID-19%20MOH.pdf?tac=eBuletin%20Ckaps/ (accessed on 24 February 2021).
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2021, 803, 149911. [Google Scholar] [CrossRef] [PubMed]
- Fernández Díez, Y.; Arenillas de la Puente, A.; Menéndez Díaz, J.Á. Microwave Heating Applied to Pyrolysis; InTech: London, UK, 2011. [Google Scholar]
- Bridgwater, A. V Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A. Development and Characterization of Polypropylene Waste from Personal Protective Equipment (PPE)-Derived Char-Filled Sugar Palm Starch Biocomposite Briquettes. Polymers 2021, 13, 1707. [Google Scholar] [CrossRef] [PubMed]
- Ahmetli, G.; Kocaman, S.; Ozaytekin, I.; Bozkurt, P. Epoxy composites based on inexpensive char filler obtained from plastic waste and natural resources. Polym. Compos. 2013, 34, 500–509. [Google Scholar] [CrossRef]
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Gonçalves, M.; Mendes, B.; Pinto, F.; Fonseca, I.; Lopes, H. Physico-chemical properties of chars obtained in the co-pyrolysis of waste mixtures. J. Hazard. Mater. 2012, 219, 196–202. [Google Scholar] [CrossRef]
- Lee, K.-H.; Noh, N.-S.; Shin, D.-H.; Seo, Y. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polym. Degrad. Stab. 2002, 78, 539–544. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deák, G.; Jover, B.; Kallo, D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis 2004, 72, 235–242. [Google Scholar] [CrossRef]
- Uddin, M.A.; Koizumi, K.; Murata, K.; Sakata, Y. Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Polym. Degrad. Stab. 1997, 56, 37–44. [Google Scholar] [CrossRef]
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of PVC I. Kinetic study. Polym. Degrad. Stab. 1999, 64, 127–144. [Google Scholar] [CrossRef]
- Sakata, Y.; Uddin, M.A.; Muto, A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis 1999, 51, 135–155. [Google Scholar] [CrossRef]
- Adrados, A.; De Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Liu, Q.; Zhang, H. Low chlorine oil production through fast pyrolysis of mixed plastics combined with hydrothermal dechlorination pretreatment. Process Saf. Environ. Prot. 2021, 149, 105–114. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Boruah, B.; Raja, D.; Vinu, R. Microwave assisted co-pyrolysis of biomasses with polypropylene and polystyrene for high quality bio-oil production. Fuel Process. Technol. 2018, 175, 64–75. [Google Scholar] [CrossRef]
- Joo, H.S.; Guin, J.A. Continuous upgrading of a plastics pyrolysis liquid to an environmentally favorable gasoline range product. Fuel Process. Technol. 1998, 57, 25–40. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Santaweesuk, C.; Janyalertadun, A. The production of fuel oil by conventional slow pyrolysis using plastic waste from a municipal landfill. Int. J. Environ. Sci. Dev. 2017, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Song, J.; Tang, T. Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide. RSC Adv. 2018, 8, 2469–2476. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Behaviour of waste polypropylene pyrolysis char-based epoxy composite materials. Environ. Sci. Pollut. Res. 2020, 27, 3871–3884. [Google Scholar] [CrossRef]
- Wong, H.-W.; Broadbelt, L.J. Tertiary resource recovery from waste polymers via pyrolysis: Neat and binary mixture reactions of polypropylene and polystyrene. Ind. Eng. Chem. Res. 2001, 40, 4716–4723. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Oochit, D. Effect of pyrolysis temperature on product yields of palm fibre and its biochar characteristics. Mater. Sci. Energy Technol. 2020, 3, 575–583. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Witkowski, A.; Stec, A.A.; Hull, T.R. Thermal decomposition of polymeric materials. In SFPE Handbook of Fire Protection Engineering; Springer: Berlin/Heidelberg, Germany, 2016; pp. 167–254. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018; ISBN 0128130407. [Google Scholar]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of pyrolysis temperature on PhysicoChemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Selvakumar, V.; Palanikumar, K.; Palanivelu, K. Studies on mechanical characterization of polypropylene/Na-MMT nanocomposites. J. Miner. Mater. Charact. Eng. 2010, 9, 671. [Google Scholar]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Bonelli, P.R.; Della Rocca, P.A.; Cerrella, E.G.; Cukierman, A.L. Effect of pyrolysis temperature on composition, surface properties and thermal degradation rates of Brazil Nut shells. Bioresour. Technol. 2001, 76, 15–22. [Google Scholar] [CrossRef]
- Norizan, M.N.; Harussani, M.M.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Basu, P. Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: Cambridge, MA, USA, 2013; pp. 87–145. [Google Scholar]
- Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I.A. Supermagnetic nano-bifunctional catalyst from rice husk: Synthesis, characterization and application for conversion of used cooking oil to biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Nurazzi, N.M.; Harussani, M.M.; Zulaikha, N.D.S.; Norhana, A.H.; Syakir, M.I.; Norli, A. Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors—An overview. Polimery 2021, 66, 85–97. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018, 128, 69–77. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene: Part III. Thermogravimetric analyses. Polym. Degrad. Stab. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Font, R.; Caballero, J.A. Thermogravimetric studies on the thermal decomposition of polyethylene. J. Anal. Appl. Pyrolysis 1996, 36, 1–15. [Google Scholar] [CrossRef]
- Kayacan, I.; Doğan, Ö.M. Pyrolysis of low and high density polyethylene. Part I: Non-isothermal pyrolysis kinetics. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 385–391. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Beerlandt, J.; Dewil, R. Thermogravimetric pyrolysis of waste polyethylene-terephthalate and polystyrene: A critical assessment of kinetics modelling. Resour. Conserv. Recycl. 2011, 55, 772–781. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, P.; Su, Z.; Wei, P.; Wang, G.; Tang, X. Synergistic effect of phosphorus, nitrogen, and silicon on flame-retardant properties and char yield in polypropylene. J. Appl. Polym. Sci. 2005, 96, 854–860. [Google Scholar] [CrossRef]
- Stuart, B. Infrared spectroscopy. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 1–18. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamental and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- UCLA, Infrared Spectroscopy Table, UCLA College. 2001. Available online: https://www.chem.ucla.edu/~bacher/General/30BL/IR/ir.html (accessed on 9 March 2021).
- Krylova, V.; Dukštienė, N. Synthesis and characterization of Ag2S layers formed on polypropylene. J. Chem. 2013, 2013, 987879. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Smidt, E.; Meissl, K. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 2007, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, X.; Ge, X.; Chen, M. Chemical pyrolysis of E-waste plastics: Char characterization. J. Environ. Manag. 2018, 214, 94–103. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, D.; Niu, T.; Yao, Y. Syntheses of two dopo-based reactive additives as flame retardants and co-curing agents for epoxy resins. Phosphorus. Sulfur. Silicon Relat. Elem. 2014, 189, 1564–1571. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S. Polyamide 6/multiwalled carbon nanotubes nanocomposites with modified morphology and thermal properties. Polymers 2013, 5, 1380–1391. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Muhammad Asyraf, M.R.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, E. Infrared and Raman spectroscopy of polypropylene. In Polypropylene; Springer: Berlin/Heidelberg, Germany, 1999; pp. 320–328. [Google Scholar]
- Bhattacharyya, A.R.; Sreekumar, T.V.; Liu, T.; Kumar, S.; Ericson, L.M.; Hauge, R.H.; Smalley, R.E. Crystallization and orientation studies in polypropylene/single wall carbon nanotube composite. Polymer 2003, 44, 2373–2377. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Young, R.J.; Kinloch, I.A. Raman spectra and mechanical properties of graphene/polypropylene nanocomposites. Int. J. Chem. Eng. Appl. 2015, 6, 1–5. [Google Scholar]
- Zhou, S.; Song, L.; Wang, Z.; Hu, Y.; Xing, W. Flame retardation and char formation mechanism of intumescent flame retarded polypropylene composites containing melamine phosphate and pentaerythritol phosphate. Polym. Degrad. Stab. 2008, 93, 1799–1806. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C. Raman “fingerprinting”of amorphous carbon films. J. Appl. Phys. 1994, 76, 3823–3830. [Google Scholar] [CrossRef]
- Lespade, P.; Al-Jishi, R.; Dresselhaus, M.S. Model for Raman scattering from incompletely graphitized carbons. Carbon N. Y. 1982, 20, 427–431. [Google Scholar] [CrossRef]

| Element | C | H | N | O | S |
|---|---|---|---|---|---|
| Amount (wt.%) | 87.85 | 10.58 | 0.23 | 0.48 | 0.86 |
| Sample | Moisture (wt.%) | Volatile Matter (wt.%) | Ash Content (wt.%) | Fixed Carbon (wt.%) |
|---|---|---|---|---|
| PP-IG | 0 | 95.75 | 0.38 | 3.87 |
| Samples | C-450 | C-500 | C-550 | C-600 |
|---|---|---|---|---|
| Temperature (°C) | 450 | 500 | 550 | 600 |
| Char yields (wt.%) | 2.27 ± 0.45 a | 2.12 ± 0.25 a,b | 2.06 ± 0.08 a,b | 1.97 ± 0.04 b |
| Samples | C-450 | C-500 | C-550 | C-600 |
|---|---|---|---|---|
| Moisture content (wt.%) | 0.53 | 0.67 | 0.50 | 0.39 |
| Volatile matter (wt.%) | 9.47 | 5.22 | 2.04 | 1.39 |
| Sample | Element Line (wt.%) | |||||
|---|---|---|---|---|---|---|
| C K | O K | Ca K | P K | Al K | K K | |
| PP-IG | 95.67 | 4.00 | 0.32 | - | - | - |
| C-450 | 27.23 | 38.87 | 22.24 | 10.55 | 0.66 | 0.46 |
| C-500 | 16.09 | 44.36 | 27.10 | 11.53 | 0.47 | 0.44 |
| C-550 | 17.45 | 46.32 | 30.53 | 4.03 | 0.60 | 0.57 |
| C-600 | 14.11 | 40.55 | 25.67 | 18.77 | 0.43 | 0.47 |
| Sample | SBET (m2 g−1) | Dp (nm) | Vp (cm3 g−1) |
|---|---|---|---|
| PP-IG | 4.98 | 28.11 | 0.0350 |
| C-450 | 17.65 | 10.86 | 0.0479 |
| C-500 | 12.81 | 20.30 | 0.0650 |
| C-550 | 25.25 | 11.75 | 0.0742 |
| C-600 | 22.91 | 14.03 | 0.0804 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harussani, M.M.; Rashid, U.; Sapuan, S.M.; Abdan, K. Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers 2021, 13, 3980. https://doi.org/10.3390/polym13223980
Harussani MM, Rashid U, Sapuan SM, Abdan K. Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers. 2021; 13(22):3980. https://doi.org/10.3390/polym13223980
Chicago/Turabian StyleHarussani, M. M., Umer Rashid, S. M. Sapuan, and Khalina Abdan. 2021. "Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char" Polymers 13, no. 22: 3980. https://doi.org/10.3390/polym13223980
APA StyleHarussani, M. M., Rashid, U., Sapuan, S. M., & Abdan, K. (2021). Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers, 13(22), 3980. https://doi.org/10.3390/polym13223980